JOINTS 2025;
3: e1731
DOI: 10.26355/joints_202512_1731
Injectable treatments in elderly patients affected by knee osteoarthritis: a systematic review of clinical evidence
Topic: Orthobiology
Category: Systematic Review
Abstract
OBJECTIVE: Intra-articular injectable therapy is used to improve the function and symptoms related to knee osteoarthritis (OA). Previous literature largely investigated the general population, while poorly focusing on older patients. The aim of this systematic review was to document the clinical evidence on intra-articular injectable treatments for elderly knee OA patients.
MATERIALS AND METHODS: A literature search was performed in May 2024 on PubMed, Cochrane, and Web of Science according to PRISMA guidelines. Studies evaluating intra-articular injectable treatments in elderly patients (≥ 65 years) were included, and data were analyzed for author, publication year, number of patients, gender, age, OA grade, concomitant treatments, injected product/protocol, safety, clinical outcomes, biomarker analyses, and imaging evaluations. The Downs and Black’s checklist was used to evaluate the risk of bias and study quality.
RESULTS: Of the 3,175 records identified in the initial search, eight studies were included. A total of 515 elderly patients (29% men and 71% women) were evaluated: 321 were treated with hyaluronic acid (HA), 182 with platelet-rich plasma (PRP), and 12 with adipose-derived stromal vascular fraction (SVF), with overall good results and limited adverse events. The Downs and Black’s checklist showed an overall poor quality of the included studies, with an average score of 15.5 ± 2.3 points (range 12-19).
CONCLUSIONS: The clinical evidence on injectable treatments for knee OA in elderly patients is limited. Although overall safety and effectiveness have been documented in this patient category for HA, PRP, and adipose-derived SVF, the number of published studies and evaluated patients is scarce, and the overall quality of evidence is low.
Introduction
Knee osteoarthritis (OA) is a common disease affecting more than 250 million people worldwide, with the elderly population being the most affected1,2. Knee OA represents a challenge for clinicians, especially in older patients, as their knees are often severely compromised and usually have a suboptimal treatment response3,4. The first-line approach remains conservative and includes self-management strategies, strengthening exercises, and low-impact aerobic activity5. When non-operative treatments fail, invasive options such as knee replacement are considered as an end-stage solution for knee OA6. Overall, good results have been reported7, especially in the elderly population, but the clinical results remain unsatisfactory in over 20% of cases. Furthermore, old patients present surgical challenges as they often suffer from several comorbidities that can complicate or even contraindicate knee replacement surgery8. For these reasons, it is important to consider alternative solutions for elderly patients affected by knee OA in order to delay and possibly avoid the need for joint replacement.
Intra-articular injectable therapy is a minimally invasive option commonly used to improve the function and symptoms related to knee OA when other conservative treatments have failed9. Increasing evidence10-13 demonstrated the clinical efficacy of knee injectable treatments in the general population affected by knee OA, with a large literature on injectable products, including corticosteroids, hyaluronic acid, platelet-rich plasma, and cell-based therapies. Even though these products are often used in clinical practice also for elderly patients, most research studies14,15 focused on patients with an average age between 55 and 65 years, often excluding older ones. Moreover, some findings16 suggested a lower efficacy for knee injection treatments in older patients compared to younger ones, probably due to a lower age-related treatment response and reparative/regenerative capacity. In this light, a recent ICRS-ESSKA consensus3 reported on the appropriateness of the use of injectable treatments not only in the general population but also in elderly patients suffering from knee OA, while recognizing the possibly lower outcomes and warning from excessive expectations. Overall, previous literature analyses largely investigated the general population, while poorly focusing on the evidence supporting the effects of injectable treatment in elderly patients, leaving the management of knee OA in this specific population a debated topic.
The aim of this systematic review was to explore the available clinical evidence on the results of intra-articular injectable treatments for elderly patients affected by knee OA.
Materials and Methods
Search Strategy and Article Selection
A literature search was performed on May 25, 2024, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, on three electronic databases (PubMed, Cochrane, and Web of Science). The following research terms were used: “(elderly OR old patients) AND (knee) AND (inject* OR intra-articular OR intra articular OR infiltration) AND (osteoarthritis OR OA)”. Studies evaluating the effectiveness of intra-articular injectable treatments in elderly patients (defined as patients ≥65 years, according to Singh and Bajorek17) were included, both those specifically focused on elderly patients and those on general populations, but that reported data on elderly patients separately. Only studies written in English were included. Case reports, or case series describing fewer than 5 cases, and articles in languages other than English were excluded. Pre-clinical, ex vivo studies, congress abstracts, and review articles were also excluded. Reference lists from the selected papers and from the systematic reviews found with the first and second screening were also considered, and all selected studies were included in the qualitative data synthesis.
Data Extraction, Assessment of Quality of Evidence
Two independent reviewers (R.R. and L.D.M.) screened all articles based on title and abstract to determine whether they met the inclusion criteria. After the first screening, the articles that met the inclusion criteria were evaluated for full-text eligibility and were selected according to the aforementioned criteria (Figure 1). In case of disagreement between the two reviewers, a third reviewer (A.B.) was consulted to reach a consensus. Data were extracted independently using Excel (Microsoft) on a data extraction form. The following data were extracted: author, year of publication, number of patients, gender, mean age, radiographic degree of OA, concomitant treatments, injected product and protocol, safety, clinical outcomes, biomarker analyses, and imaging evaluations.
The Downs and Black’s “checklist for measuring quality” was used to evaluate the quality of the included studies18. This checklist contains 27 ‘yes’-or-‘no’ questions across five sections, providing a numeric value up to 32 points. The five sections include questions about the study’s overall quality (10 items), the ability to generalize findings (3 items), the study bias (7 items), the confounding and selection bias (6 items), and the power of the study (1 item). Assessment of risk of bias and quality of evidence was completed independently for all outcomes by two authors (L.D.M and R.R), and a third author (A.B.) solved possible discrepancies.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. Unrelated: articles focused on elderly patients or older adults, but not on intra-articular knee injections.

Results
Article Selection and Characteristics
After duplicates were removed, the initial search identified 3,175 records, whose abstracts were screened and selected according to the inclusion/exclusion criteria for a total of 113 articles assessed for eligibility. After full-text evaluation, 78 studies were excluded as they did not provide specific data on elderly patients, 23 were unrelated articles not concerning intra-articular injectable treatments of the knee, 2 were reviews, and 2 were case reports. Thus, a total of 8 clinical studies19-26 focusing on intra-articular injectable treatments for the management of knee OA in elderly patients were included in this systematic review. The first article was published in 2001, and there has been no increase in publications over the years, except for a peak of four articles published in the 2016-2020 five-year period with a focus on orthobiologic injectable options (Figure 2).
Figure 2. Number of articles published over time on injectable therapies for knee osteoarthritis in elderly patients. HA, Hyaluronic Acid, PRP, platelet-rich plasma, SVF, stromal vascular fraction.
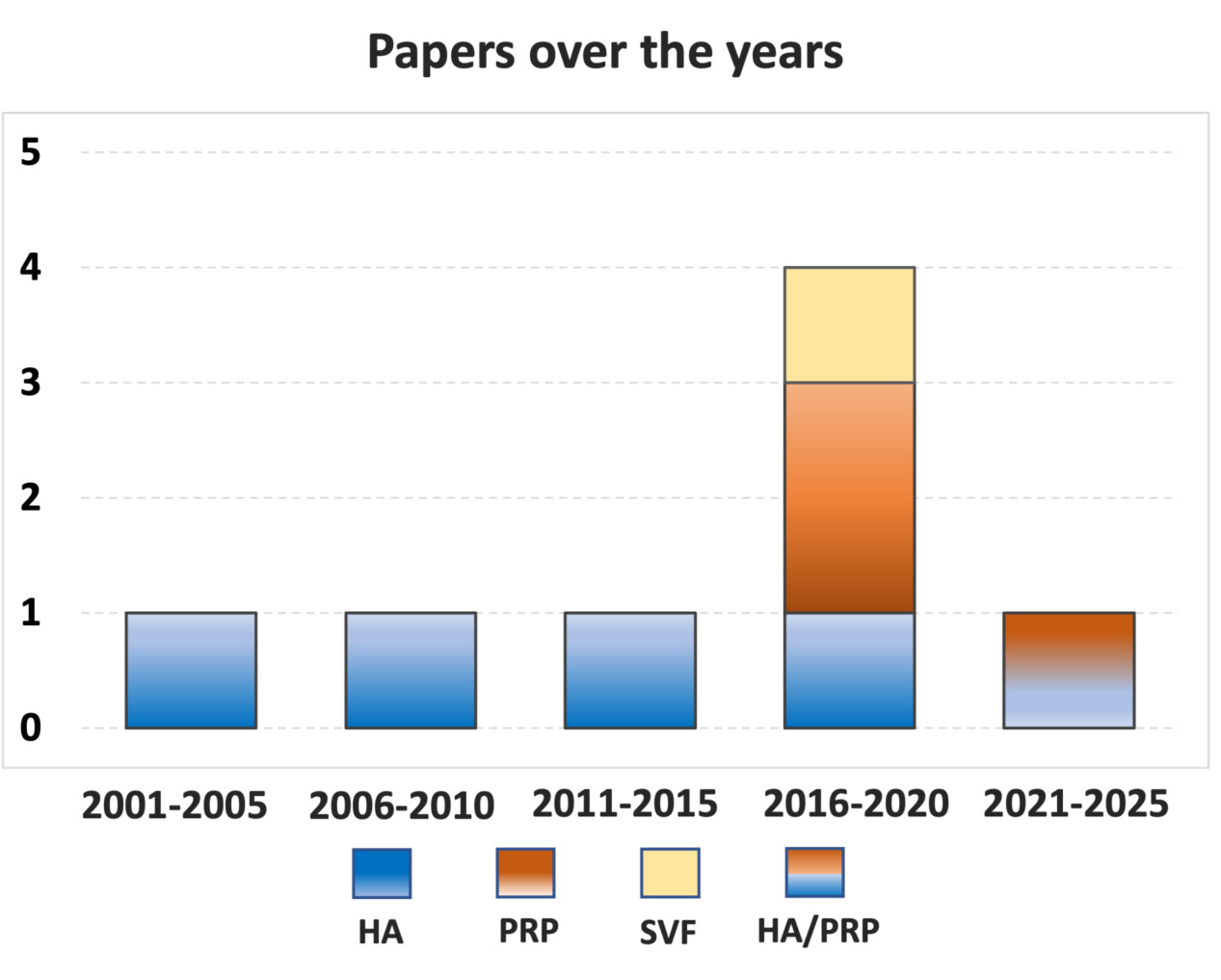
Among the included studies, the evaluation by study type showed five prospective case series19,21-24, one randomized controlled trial (RCT)20, one retrospective case series26, and one retrospective comparative study25. Three injectable products were investigated: four studies focused on intra-articular injections of hyaluronic acid (HA)19-22, two studies focused on intra-articular injections of platelet-rich plasma (PRP)23,24, one study analyzed the comparison between HA and PRP25, and one study focused on intra-articular injections of adipose-derived stromal vascular fraction (SVF)26. A total of 515 elderly (≥ 65 years) patients (29% men and 71% women) treated with intra-articular injections were evaluated: 321 were treated with HA (91 men and 230 women), 182 with PRP (105 men and 77 women), and 12 with adipose-derived SVF (men and women not specified).
The severity of OA was defined with the Kellgren-Lawrence (KL) grade in five studies19-21,25,26, while the other three studies22-24 utilized the Ahlbäck classification. The trial duration was heterogeneous among studies, ranging from 4 weeks to 7 years of follow-up, with an average of 16.6 ± 27.5 months. The visual analogue scale (VAS) for pain (four articles22,23,25,26), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, three articles19,25,26), and the Lequesne index (three articles22,24,26) were the most used scores. Other scores, such as the International Knee Documentation Committee (IKDC), the EuroQol Visual Analog Scale (EQ-VAS), and the WOMAC subscales pain and stiffness, were used in fewer than three articles. Among the included articles, two studies21,24 evaluated the synovial fluid biomarkers, and one study22 evaluated the balance of the patients through balance tests. The number of injections was heterogeneous among studies, ranging from one to five. Two studies25,26 used a single injection (SVF and a comparison HA vs. PRP), three studies19,23,24, (one on HA and two on PRP) evaluated the results of an injection cycle of three injections, while three studies20-22 on HA evaluated the results of an injection cycle of five injections. Further characteristics of the included studies and the injected products are reported in Table 1. A synthesis of the overall results of the studies included is reported in Table 2, while more details on each study are reported in the following paragraphs.
The evaluation using the Downs and Black checklist showed poor overall quality of the included studies, with an average score of 15.5 ± 2.3 points (range 12-19), as reported in Table 3.
Table 1. Characteristics of the included studies.
| Authors | Study design | Inclusion criteria | N° of patients (M/F) Mean Age (y) |
Concomitant treatment | Injected product | Injection protocol (volume, n° injections, interval) | Follow-ups Evaluations |
Main findings |
| Herrero-Beaumont et al21 2001 |
Prospective case series | Kellgren-Lawrence IV, No injective treatments in the past 6 months | 20 (2/18) 70.9 [65-82] |
Knee aspiration | Hyaluronic acid (Adant, Meiji-Seika Kaisha in Japan and Tedec-Meiji in Spain) 900 kDa | 25 mg/2.5 ml, 5 injections, 1-week interval |
Baseline, 4 w, cartilage and bone biomarkers | Sodium hyaluronate could increase cartilage and bone metabolism due to the increased overuse of the joint caused by the analgesic effect of sodium hyaluronate. No side effects. |
| Sun et al22 2006 |
Prospective case series | Unilateral symptomatic knee OA > 6 months, VAS ≥ 3 and radiographic OA (Ahlbäck I-II) | 56 (35/21) 74.7 ± 5.4 |
None | Hyaluronic acid (Artzal, Seikagaku Corp., Japan)
900 kDa |
2.5 mL, 5 injections, 1-week interval |
Baseline, 1 w, 1 m, 3 m, 6 m VAS, Lequesne index and four balance tests scores |
Five weekly intraarticular injections of HA produced a reduction in pain, significant improvement in physical function and clinical tests of balance.
Local adverse events (pain, local warmth and swelling) reported in 4 patients. No severe adverse events. |
| Uçar et al19 2013 |
Prospective case series | Kellgren-Lawrence II-III | 71 (15/56) 71.3 [65-84] |
None | Hyaluronic acid (NR)
NR kDa |
30 mg/ml, 3 injections, 1-week interval |
Baseline, 1 m, 12 m VAS resting pain, VAS activity pain, WOMAC |
Improvement in pain relief and function after 1 month, no improvement compared to baseline at 12 months of follow-up. No side effects. |
| Ip and Fu20 2015 |
Randomized controlled trial | Bilateral radiographic osteoarthritis (Kellgren-Lawrence III), synovitis and pain in both knees | 70 (20/50) 75 [70-80] |
Laser therapy | Hyaluronic acid (Hyalgan, Fidia, Italy)
500-730 kDa |
20 mg/2 ml, 5 injections, 1-week interval every 6 months |
Baseline, 7 y WOMAC pain, WOMAC stiffness |
Higher conversion to knee replacement for the untreated knee compared to the treated one. No side effects. |
| Bottegoni et al23 2016 |
Prospective case series | Hematologic blood dyscrasias with platelet dysfunction, anemia, unilateral knee OA, ≥ 4 months pain or swelling, limitation of daily activities, and radiographic OA (Ahlbäck I-III) | 60 (21/39) 72 ± 5.9 |
None | Homologous PRP | 5 mL, 3 injections, 2-week interval |
Baseline, 2 m, 6 m IKDC, KOOS, EQ-VAS |
PRP has an excellent safety profile and offers clinical improvement in elderly patients. High-grade joint degeneration results in a decreased potential for homologous PRP injective therapy. Burning sensation or mild pain in 9 patients. |
| Chen et al24 2017 |
Prospective case series | History of failed previous conservative treatments, suprapatellar bursitis due to knee OA, knee pain or swelling > 6 months and image findings of knee OA (Ahlbäck I-III) | 24 (10/14) 70 ± 3.1 |
Knee aspiration | Fresh autologous PRP | 5 mL, 3 injections, 1-months interval |
Baseline, 2° injection, 3° injection, 3 m, 6 m SF volume, inflammatory proteins, Lequesne index |
After PRP treatment inflammatory proteins decreased and proteins associated with anti-aging physiological functions increased significantly. These changes were combined with clinical improvements of decreased SF volumes and index of OA severity. Side effects not reported. |
| Lapuente et al26 2020 | Retrospective case series | Clinical and radiological signs of bilateral knee OA, Kellgren-Lawrence grade III or IV. History of failed previous conservative treatments | 12 (NR) / [70-89] |
Knee aspiration and SVF injection in the Hoffa’s fat pad | Enzymatic digestion SVF Autologous (ADSC System commercial kit, Lyposmol Biotech, Madrid, Spain) | 7 mL. 1 injection, / |
Baseline, 3 m, 6 m, 12 m VAS, Lequesne, WOMAC |
Clinical improvement from baseline up to 12 months of follow-up. Age of the patients did not influence the clinical outcome. Adverse reactions observed were mild and transient (abdominal discomfort related with liposuction procedure). No severe adverse events in elderly patients. |
| Pamuk25 2022 |
Retrospective comparative study | Kellgren-Lawrence II-III, VAS ≥ 40, failure of pharmacologic treatment | 202 (43/159) 72.5 ± 5.4 |
None | Hyaluronic Acid (Monovisc, DePuy Synthes, USA)
1,000-2,900 kDa
Fresh autologous PRP |
20 mg/4 mL HA
4 mL PRP 1 injection |
Baseline, 1 m, 3 m, 6 m IKDC, VAS, WOMAC |
Intra-articular PRP and HA injections were both effective treatment for geriatric patients with knee OA, as demonstrated by the observed pain-related and functional improvements, particularly within the first 3 months. No severe adverse events. |
F = Female, HA = Hyaluronic acid, IKDC = International Knee Documentation Committee Subjective Knee Form, y = Years, kDA, kilo daltons; KOOS = Knee injury and Osteoarthritis Outcome Score, M = Male, m = months, NR = not reported, OA = Osteoarthritis, PRP = Platelet-rich plasma, sd = Standard Deviation, SF = Synovial Fluid, SVF = Stromal vascular fraction, VAS = Visual analogic scale, w = weeks, WOMAC = Western Ontario and McMaster University Osteoarthritis index.
Table 2. Synthesis of the overall results of the included studies.
| Article | Product | Clinical outcome | Biomarkers analysis | Imaging evaluation |
| Herrero-Beaumont et al21 2001 | HA | / | + | / |
| Sun et al22 2006 | HA | + | / | / |
| Uçar et al19 2013 | HA | + | / | / |
| Ip and Fu20 2015 | HA | + | / | / |
| Pamuk25 2022 | HA | + | / | / |
| PRP | + | / | / | |
| Chen et al24 2017 | PRP | + | + | / |
| Bottegoni et al23 2016 | PRP | + | / | / |
| Lapuente et al26 2020 | Adipose-SVF | + | / | / |
HA = Hyaluronic Acid, PRP = platelet-rich plasma, SVF = stromal vascular fraction. Outcome (+) positive, (/) not analyzed, (-) negative.
Table 3. Methodological quality of the included studies with the risk of bias evaluation.
| Articles | Reporting | External validity | Internal validity bias | Internal validity confounding | Power | Total |
| Herrero-Beaumont et al21 2001 | 9 | 1 | 4 | 1 | 0 | 15 |
| Sun et al22 2006 | 9 | 3 | 5 | 1 | 0 | 18 |
| Uçar et al19 2013 | 9 | 2 | 4 | 1 | 0 | 16 |
| Ip and Fu20 2015 | 9 | 1 | 5 | 4 | 0 | 19 |
| Bottegoni et al23 2016 | 9 | 1 | 5 | 1 | 0 | 16 |
| Chen et al24 2017 | 7 | 1 | 4 | 0 | 0 | 12 |
| Lapuente et al26 2020 | 7 | 1 | 5 | 1 | 0 | 14 |
| Pamuk25 2022 | 8 | 1 | 4 | 1 | 0 | 14 |
HA Injections
Four studies19-22 specifically focused on intra-articular HA injections in elderly patients. Three studies20-22 evaluated low molecular weight HA: Hyalgan (Fidia Farmaceutici, Abano Terme, Italy, 500-730 kDA), Adant (Meiji–Seika Kaisha, Tokyo, Japan and Tedec–Meiji, Alcala de Henares, Spain, 900 kDa), and Artzal (Seikagaku Corp., Tokyo, Japan, 900 kDa). The fourth study19 did not report details about the HA molecular weight.
– The prospective placebo-controlled RCT by Ip and Fu20 analyzed 70 elderly patients [mean age 75 (70-80)] with bilateral knee OA treated with 5-weekly injections of Hyalgan and low-level laser therapy in the knee and saline injection and simulated laser therapy in the contralateral knee on a half-year basis. The authors reported a lower conversion to knee replacement for the knees treated with HA and low-level laser therapy compared to the contralateral one (1 knee HA group vs. 15 knees placebo group) at a mean follow-up of 7 years.
– The study of Sun et al22 evaluated 56 elderly patients (mean age 74.7 ± 5.4) affected by knee OA and treated with 5-weekly injections of Artzal, reporting a significant improvement of VAS and Laquesne index after 1 week and a lasting effect up to 6 months. Moreover, patients underwent clinical balance tests demonstrating a balance improvement after the treatment. The authors also evaluated the demand for analgesics (acetaminophen) before and after the injectable treatment, reporting a significant decrease in the use of analgesics after HA injections.
– The study by Uçar et al19 analyzed 172 patients affected by knee OA and treated with 3-weekly knee injections. The authors subdivided the sample into a “middle-aged group” (age <65 years, 101 patients) and an “elderly group” (age ≥ 65 years, mean age 71.3, range 65-84, 71 patients), analyzing both groups separately. The “elderly group” reported significant clinical improvements in terms of VAS and WOMAC score at 1 month of follow-up, without a lasting effect at 12 months of follow-up.
– The study by Herrero-Beaumont et al21 evaluated 20 elderly patients [mean age 70.9 (65-82)] affected by knee OA treated with 5 intra-articular knee injections of Adant. The authors collected and analyzed synovial fluid and urine samples at the first and last knee injections, showing a significant increase in cartilage degradation and bone markers in synovial fluid levels at the last injection compared to the first, which they interpreted as a positive sign related to increased physical activity.
Regarding the safety of HA injectable treatments for elderly patients, none of the included studies reported severe adverse events, while only one study22 reported self-limiting local adverse events such as pain, local warmth, and swelling in 4 patients, for an overall adverse events rate of 1.8% (4/217 patients).
PRP Injections
Two studies specifically focused on intra-articular injections of PRP in elderly patients.
– The study of Bottegoni et al23 analyzed 60 elderly patients (mean age 72.0 ± 5.9) affected by symptomatic knee OA treated with a single injection of PRP (5 mL) and evaluated at 2 and 6 months. PRP was homologous, fresh, poor in leukocytes, with a concentration of 1,200-1,600 × 103/μl, and activated with 10% calcium chloride. The authors reported a significant improvement in terms of IKDC subjective score, KOOS, and EQ-VAS at 2 and 6 months compared to baseline, while clinical worsening was documented at 6 months compared to 2 months of follow-up. Moreover, better clinical outcomes were observed in patients aged 65-79 and in patients with lower-grade joint degeneration (Ahlbäck I-II) compared with patients >80 or with higher-grade joint degeneration (Ahlbäck III).
– The study of Chen et al24 reported on 24 elderly (mean age 70.0 ± 3.1) patients affected by knee OA treated with three monthly PRP injections (5 mL) and evaluated at up to 6 months of follow-up. PRP was autologous and without external activation, while other product characteristics were not documented. This study evaluated patients through the Lequesne index, quantification of the synovial fluid, and synovial fluid biomarker analysis, reporting after the second monthly PRP injection a reduction in the inflammatory proteins, with a significant decrease in synovial fluid volume and Lequesne index.
Regarding the safety of PRP injections in elderly patients, only one of these studies23 reported adverse events, documenting the presence of 9 patients with burning sensations or mild pain after treatment (adverse event rate of 15%). No severe adverse events were documented. The other study did not report any data regarding the safety of the injectable treatment.
HA vs. PRP Injections
Only one comparative retrospective study investigated the clinical results of different injectable treatments in elderly patients.
– Pamuk25 retrospectively evaluated elderly patients (mean age 72.5 ± 5.4) affected by knee OA (KL 2-3) who underwent a single knee injection of HA or PRP (104 and 98 patients, respectively). The authors used a medium-weight HA (Monovisc, 20 mg/4 ml). PRP was autologous, while platelet/leukocyte concentration and PRP activation method were not reported. Both injectable treatments proved to be effective in terms of clinical improvement at all follow-ups. The HA group showed a significantly greater improvement compared to the PRP group in terms of VAS and IKDC scores at 3 months compared to baseline, although these differences disappeared at 6 months of follow-up.
No serious adverse events were reported after both injectable treatments.
Adipose-Derived SVF
Only one retrospective study26 reported the results of adipose-derived SVF injectable treatment in elderly patients.
– This study analyzed patients affected by knee OA (KL 3-4) treated with a single intra-articular and intra-Hoffa’s fat pad injection of adipose-derived SVF (7 mL). The kit utilized was the ADSC System commercial kit (Lyposmol Biotech, Madrid, Spain), which involved the use of enzymatic digestion of the adipose tissue with collagenase I and II and a subsequent centrifugation to obtain the SVF. The authors reported clinical data stratified by patient age, showing a significant improvement at 12 months of follow-up in VAS, Lequesne, and WOMAC scores compared with baseline in the subgroup of 12 elderly patients (ages 70-89) treated with adipose-derived SVF.
Regarding safety, mild and transient adverse events (abdominal discomfort related to the liposuction procedure) were reported without specifying the exact number of patients, while no severe complications were documented.
Discussion
The main finding of this systematic review is that the available clinical evidence on the use of injectable treatments for knee OA in elderly patients is very limited. Although overall safety and effectiveness have been documented in this patient category for HA, PRP, and adipose-derived SVF, the number of published studies and evaluated patients is scarce, and the overall quality of evidence is low. This makes it difficult to establish the real clinical benefit offered by injectable treatments in elderly patients with knee OA.
This systematic review highlighted the scarce attention paid to the investigation of injectable treatments for elderly patients, which does not reflect the broader evidence on their use in the general population13. The number of specific studies focusing on elderly patients affected by knee OA is limited, with fewer than 600 patients analyzed across the existing literature. Furthermore, this literature review revealed a poor level of evidence and quality of the available studies. To date, clinical studies on elderly patients are mostly prospective or retrospective case series involving a low number of patients, while there is only one small placebo-controlled RCT. However, this study used another conservative treatment in addition to intra-articular HA injectable therapy, impairing the possibility of establishing the specific contribution and the real efficacy of the injectable product compared to the placebo effect. The placebo effect plays an important role in injectable treatments, especially in the case of new interesting products27. The contribution of the placebo effect has been demonstrated to be particularly relevant in terms of pain reduction after intra-articular injections in patients affected by knee OA, with a clinically relevant improvement reported up to 6 months. In this scenario, a significant placebo effect could also be present in elderly patients treated with intra-articular injectable treatments. Therefore, specific double-blind placebo-controlled RCTs are needed to establish the real benefit offered by these injectable treatments in elderly patients, since only treatments that statistically and clinically overcome the placebo effect should be recommended in clinical practice.
This systematic review documented clinical studies focusing on three different products used for the injectable treatment of knee OA in elderly patients: HA, PRP, and adipose-derived SVF. The available evidence supporting the use of adipose-derived SVF in knee OA elderly patients consists of a subgroup of patients in a single small retrospective study26. With this in mind, it is impossible to establish the clinical efficacy of this type of product. Although still limited, more evidence is available for the other two types of products analyzed, HA and PRP, respectively. Viscosupplementation showed not only a clinical improvement but also a reduction in the conversion to knee replacement surgery and an improvement in the balance of patients, an extremely important aspect, especially in managing elderly patients, where the risk of fall is extremely high28. However, the four studies19-22 reporting HA injections are heterogeneous in terms of evaluated products, with different molecular weights, volumes, and injection protocols, making it difficult to draw clear conclusions regarding the benefits of these products. Similarly, the two available studies23,24 on PRP showed encouraging clinical results, although these studies presented heterogeneity in terms of type of PRP, injection protocols, activation method, and production technique. All these differences should be explored in specific clinical trials to identify the parameters that could optimize this orthobiological injectable treatment for the management of knee OA in elderly patients.
Only one comparative study25 investigated the role of HA and PRP injectable treatments in elderly patients. This trial reported interesting findings, demonstrating that a single intra-articular injection of HA provided better results compared to a single intra-articular injection of PRP in elderly patients at 3 months of follow-up, although the differences between the two groups were not confirmed at 6 months. These data are surprising considering the overall literature on injectable treatments for patients affected by OA. Several meta-analyses9,29,30 showed the superiority of PRP injections compared to HA in the general population with knee OA, with better clinical outcomes provided by PRP, especially at longer follow-up. Future high-level studies should compare the clinical results obtained over time from HA and PRP injectable treatments, as well as with a placebo control and with the most commonly used corticosteroid injectable therapy.
The current analysis of the literature also highlighted the lack of data on the safety and efficacy of intra-articular corticosteroid injection in elderly patients affected by knee OA. This data is surprising, taking into account that the use of corticosteroid injections in elderly patients is not only common in clinical practice but is also recommended by some guidelines of scientific societies for the treatment of knee OA31. Nevertheless, a recent meta-analysis10 evaluating placebo-controlled RCT on knee OA patients highlighted that intra-articular corticosteroid injections offer clinically perceivable pain relief and functional improvement higher than the placebo effect only at short-term follow-up, with benefits losing clinical relevance already after 6 weeks27. The short-term effectiveness of corticosteroids was also confirmed in another meta-analysis32, demonstrating how corticosteroid injections for knee OA offer comparable results to HA and PRP only at short-term follow-up, while lower results compared to PRP at longer follow-up. Further studies are needed to investigate, specifically, whether these results will be confirmed in elderly patients with knee OA, evaluating the safety profile and clinical efficacy of corticosteroids in these patients.
The limitations of this systematic review reflect the limitations of the literature. The analysis revealed that the current clinical evidence is insufficient and largely characterized by a low level of quality. The studies currently available exhibit significant heterogeneity in terms of injected products and injection protocols. Furthermore, the studies often failed to provide precise and consistent reports on the number and nature of adverse events, frequently utilizing heterogeneous definitions, hindering the possibility of obtaining an accurate rate of adverse events. Finally, there is not enough stratified and homogeneous data based on the type of injected product, making it difficult to merge and compare clinical results, thus impairing the possibility of performing a reliable meta-analysis to draw clear conclusions on the clinical efficacy of these products. Future studies should better analyze clinical outcomes by stratifying by product and patient characteristics based on their age and functional demands, and confirm the preliminary positive results that emerged from the current literature analysis by comparing the products used also with a placebo in well-designed RCTs. These and other characteristics of patients and included products should be evaluated to improve the management of elderly patients with knee OA. Nevertheless, despite the aforementioned limitations, this systematic review offered a comprehensive picture of the state of the art in the field, highlighting safety and overall promising clinical outcomes. However, considering the limitations of the available literature, the increasing support for the use of these treatments in clinical practice in elderly patients does not seem to be sufficiently supported by the current scientific evidence, especially as a first-line treatment. However, given the favorable safety profile even in this specific population, as reported by this systematic review, injectable treatment could be considered appropriate in selected cases, particularly in patients who did not respond to conservative treatment or patients who are unwilling or unsuitable for joint replacement surgery. Moreover, when injectable treatments are indicated, the choice of the product to be injected should be based on available evidence and scientific societies’ guidelines for these products, as studies specifically involving older adults do not allow for drawing definitive conclusions. Therefore, further high-level studies are needed to better clarify the real therapeutic potential, the most suitable indications, the optimal product and approach to use intra-articular injectable treatments to better manage elderly patients affected by knee OA.
Conclusions
The available clinical evidence on the use of injectable treatments for knee OA in elderly patients is very limited. Although overall safety and effectiveness have been documented in this patient category for HA, PRP, and adipose-derived SVF, the number of published studies and evaluated patients is scarce, and the overall quality of evidence is low. This makes it difficult to establish the real clinical benefit offered by injectable treatments in elderly patients with knee OA.
Ethics Approval and Informed Consent:
No Ethical Committee approval or patient consent was needed due to the nature of the study.
Availability of Data and Materials:
All data generated or analyzed during this study are included in this manuscript.
Conflict of Interest:
S.Z. reports non-financial support from personal fees from I + SRL and grants from Fidia Farmaceutici SPA, Cartiheal Ltd., IGEA clinical biophysics, BIOMET, and Kensey Nash, outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. The other authors declare no conflict of interest.
Funding:
This study was funded by the Italian Ministry of Health in the Project “Giovane Ricercatore” “Bone Marrow Vs Adipose Tissue: A Comparison of Mesenchymal Stromal Cells Concentrates for Knee Osteoarthritis Treatment – MAST” (GR-2021-12374140, PI Dr. Luca Andriolo).
Authors’ Contributions:
Conceptualization, G.F.; methodology, L.D.M., A.Bo, R.R; data curation, L.D.M. and R.R.; writing–original draft preparation, L.D.M. and A.Bo.; writing–review and editing, S.M., L.A., and G.F.; supervision, G.F. and S.Z. All authors have read and agreed to the published version of the manuscript.
ORCID ID:
L. De Marziani: 0000-0002-3714-058X
A. Boffa: 0000-0002-1523-6900
G. Filardo: 0000-0002-8136-0977
L. Andriolo: 0000-0001-6352-9671
AI Disclosure:
During the preparation of this work, the authors did not use any AI or AI-assisted technologies to improve the scientific work.
References
- Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020; 29-30
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1323-1330.
- Kon E, de Girolamo L, Laver L, Andriolo L, Andia I, Bastos R, Beaufils P, Biant L, Bøe B, Boffa A, Cugat R, Di Martino A, Erggelet C, Iosifidis M, Kocaoglu B, Magalon J, Marinescu R, Nehrer S, Niemeyer P, Ostojić M, Piontek T, Sánchez M, Sas K, Skarpas G, Tischer T, Vonk L, Filardo G. Platelet-rich plasma injections for the management of knee osteoarthritis: The ESSKA-ICRS consensus. Recommendations using the RAND/UCLA appropriateness method for different clinical scenarios. Knee Surg Sports Traumatol Arthrosc 2024; 32: 2938-2949.
- Laver L, Filardo G, Sanchez M, Magalon J, Tischer T, Abat F, Bastos R, Cugat R, Iosifidis M, Kocaoglu B, Kon E, Marinescu R, Ostojic M, Beaufils P, de Girolamo L; ESSKA‐ORBIT Group. The use of injectable orthobiologics for knee osteoarthritis: A European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma). Knee Surg Sports Traumatol Arthrosc 2024; 32: 783-797.
- Parker DA, Scholes C, Neri T. Non-operative treatment options for knee osteoarthritis: current concepts. JISAKOS 2018; 3: 274-281.
- Schmitt J, Lange T, Günther KP, Kopkow C, Rataj E, Apfelbacher C, Aringer M, Böhle E, Bork H, Dreinhöfer K, Friederich N, Frosch KH, Gravius S, Gromnica-Ihle E, Heller KD, Kirschner S, Kladny B, Kohlhof H, Kremer M, Leuchten N, Lippmann M, Malzahn J, Meyer H, Sabatowski R, Scharf HP, Stoeve J, Wagner R, Lützner J. Indication Criteria for Total Knee Arthroplasty in Patients with Osteoarthritis – A Multi-perspective Consensus Study. Z Orthop Unfall 2017; 155: 539-548.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res 2010; 468: 57-63.
- Kennedy JW, Johnston L, Cochrane L, Boscainos PJ. Total knee arthroplasty in the elderly: does age affect pain, function or complications? Clin Orthop Relat Res 2013; 471: 1964-1969.
- Qiao X, Yan L, Feng Y, Li X, Zhang K, Lv Z, Xu C, Zhao S, Liu F, Yang X, Tian Z. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord 2023; 24: 926.
- Belk JW, Lim JJ, Keeter C, McCulloch PC, Houck DA, McCarty EC, Frank RM, Kraeutler MJ. Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy 2023; 39: 1714-1734.
- Vilchez-Cavazos F, Blázquez-Saldaña J, Gamboa-Alonso AA, Peña-Martínez VM, Acosta-Olivo CA, Sánchez-García A, Simental-Mendía M. The use of platelet-rich plasma in studies with early knee osteoarthritis versus advanced stages of the disease: a systematic review and meta-analysis of 31 randomized clinical trials. Arch Orthop Trauma Surg 2023; 143: 1393-1408.
- Jawanda H, Khan ZA, Warrier AA, Acuña AJ, Allahabadi S, Kaplan DJ, Ritz E, Jackson GR, Mameri ES, Batra A, Dornan G, Westrick J, Verma NN, Chahla J. Platelet-Rich Plasma, Bone Marrow Aspirate Concentrate, and Hyaluronic Acid Injections Outperform Corticosteroids in Pain and Function Scores at a Minimum of 6 Months as Intra-Articular Injections for Knee Osteoarthritis: A Systematic Review and Network M. Arthroscopy 2024; 40: 1623-1636.e1.
- Singh H, Knapik DM, Polce EM, Eikani CK, Bjornstad AH, Gursoy S, Perry AK, Westrick JC, Yanke AB, Verma NN, Cole BJ, Chahla JA. Relative Efficacy of Intra-articular Injections in the Treatment of Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Am J Sports Med 2022; 50: 3140-3148.
- Gobbi A, Lad D, Karnatzikos G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2015; 23: 2170-2177.
- Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 2016; 17: 67.
- Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011; 19: 528-535.
- Singh S, Bajorek B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm Pract (Granada) 2014; 12: 489.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377-384.
- Uçar D, Dıraçoğlu D, Süleyman T, Capan N. Intra-articular hyaluronic Acid as treatment in elderly and middle-aged patients with knee osteoarthritis. Open Rheumatol J 2013; 7: 38-41.
- Ip D, Fu NY. Can combined use of low-level lasers and hyaluronic acid injections prolong the longevity of degenerative knee joints? Clin Interv Aging 2015; 10: 1255-1258.
- Herrero-Beaumont G, Guerrero R, Sánchez-Pernaute O, Acebes C, Palacios I, Mas S, Rodriguez I, Egido J, Vivanco F. Cartilage and bone biological markers in the synovial fluid of osteoarthritic patients after hyaluronan injections in the knee. Clin Chim Acta 2001; 308: 107-115.
- Sun SF, Hsu CW, Hwang CW, Hsu PT, Wang JL, Tsai SL, Chou YJ, Hsu YW, Huang CM, Wang YL. Hyaluronate improves pain, physical function and balance in the geriatric osteoarthritic knee: a 6-month follow-up study using clinical tests. Osteoarthritis Cartilage 2006; 14: 696-701.
- Bottegoni C, Dei Giudici L, Salvemini S, Chiurazzi E, Bencivenga R, Gigante A. Homologous platelet-rich plasma for the treatment of knee osteoarthritis in selected elderly patients: an open-label, uncontrolled, pilot study. Ther Adv Musculoskelet Dis 2016; 8: 35-41.
- Chen CPC, Cheng CH, Hsu CC, Lin HC, Tsai YR, Chen JL. The influence of platelet rich plasma on synovial fluid volumes, protein concentrations, and severity of pain in patients with knee osteoarthritis. Exp Gerontol 2017; 93: 68-72.
- Pamuk Ç. Comparison of Intra-articular Hyaluronic Acid and Platelet-Rich Plasma Injection in Knee Osteoarthritis: Do the Results Differ in Geriatric Patients? A Retrospective Observational Study. Ann Geriatr Med Res 2022; 26: 340-346.
- Lapuente JP, Dos-Anjos S, Blázquez-Martínez A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res 2020; 15: 137.
- Bensa A, Albanese J, Boffa A, Previtali D, Filardo G. Intra-articular corticosteroid injections provide a clinically relevant benefit compared to placebo only at short-term follow-up in patients with knee osteoarthritis: A systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2024; 32: 311-322.
- Coulter JS, Randazzo J, Kary EE, Samar H. Falls in Older Adults: Approach and Prevention. Am Fam Physician 2024; 109: 447-456.
- Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Sports Med 2021; 49: 249-260.
- Tang JZ, Nie MJ, Zhao JZ, Zhang GC, Zhang Q, Wang B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res 2020; 15: 403.
- Brophy RH, Fillingham YA. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J Am Acad Orthop Surg 2022; 30: e721-e729.
- Bensa A, Sangiorgio A, Boffa A, Salerno M, Moraca G, Filardo G. Corticosteroid injections for knee osteoarthritis offer clinical benefits similar to hyaluronic acid and lower than platelet-rich plasma: a systematic review and meta-analysis. EFORT Open Rev 2024; 9: 883-895.
To cite this article
Injectable treatments in elderly patients affected by knee osteoarthritis: a systematic review of clinical evidence
JOINTS 2025;
3: e1731
DOI: 10.26355/joints_202512_1731
Publication History
Submission date: 05 May 2025
Revised on: 09 Jul 2025
Accepted on: 03 Dec 2025
Published online: 23 Dec 2025