JOINTS 2023;
1: e639
DOI: 10.26355/joints_20236_639
The use of endogenous diathermy therapy for pain and swelling after total knee arthroplasty: comparison of high frequency vs. low frequency treatment
Topic: Knee
Category: Original article
Abstract
OBJECTIVE: The onset of edema and pain after Total Knee Arthroplasty (TKA) affects functional recovery. Endogenous diathermy therapy (EDT) has a pain-relieving effect and acts on microcirculation to reduce swelling. Different EDT devices deliver electromagnetic waves at different frequencies. The aim of our retrospective observational study was to evaluate whether High-frequency treatment (HFT) devices (>2 MHz) have a different impact on pain and swelling reduction after TKA compared with low-frequency treatment (LFT) devices (<2 MHz).PATIENTS AND METHODS: Among patients admitted for post-TKA rehabilitation, 33 subjects were evaluated for HFT and 34 subjects for LFT. Outcome measures were: limb circumferences and Numerical Rating Scale (primary outcomes); degrees of knee flexion, Timed Up and Go test and the level of pharmacotherapy used (secondary outcomes). Participants were assessed at T0 (patients entering the rehabilitation setting, four days after surgery), T1 (mid-time of the rehabilitation program, 14 days after surgery) and T2 (24 days after surgery, before hospital discharge). In order to evaluate changes over time and between groups, linear mixed model analyses for repeated measures (p=0.05) were made of each of the outcome measures.
RESULTS: Subjects of the two groups did not differ in terms of any demographic or anthropometric parameter (Kolmogorov-Smirnov test). At T2, participants of the two groups did not differ in terms of level of pharmacotherapy used (Mann-Whitney U test). A significant effect of time was found for all outcomes, none of the outcomes showed group effect and/or time-group interaction effect, resulting for both groups a progressive reduction of pain measured by NRS scale, progressive reduction of limb circumferences, progressive improvement in knee flexion degrees and progressive better performance at the TUG test throughout the rehabilitation recovery.
CONCLUSIONS: Results suggest that there is no difference in terms of EDT efficacy on postoperative pain and edema reduction using different frequencies.
Introduction
The number of primary Total Knee Arthroplasty (TKA) increased approximately by 13% in the last decade in Europe1. According to the latest available annual reports, the number of primary TKA recorded in national European registries is 2.5 million2 and its incidence rate is projected to increase by around 43% over the next 30 years3.
As the demand for surgery increases, postoperative management has to be more effective and efficient. In fact, the onset of edema and pain after TKA is a relevant problem which affects functional recovery and requires an early multidimensional intervention to be solved4,5. For this reason, good clinical practice suggests the use of electrical therapies6,7 in association with physiotherapy8, in order to reduce drugs’ overuse and side effects. Among them, endogenous diathermy therapy (EDT) has a primary role. This therapy uses radiofrequencies to generate heat in the treatment area, both on surface and in deep tissues9-11. Heat has a pain-relieving effect and acts on microcirculation modifying impedance of tissues treated. EDT has a documented capacity to reduce swelling and pain in several musculoskeletal and lymphatic disorders12-16. Its use in postoperative phase is safe17 and it is not in contrast with cryotherapy, which is largely used in immediate and early postoperative management18. As suggested by major device producers, the use of EDT is limited by contraindications directly related to radiofrequencies and to heat itself. They include: presence of pacemaker implant, implantable cardioverter defibrillator or any other cardiac implant, neoplasia, local acute infections, history of epilepsy, pregnancy, thrombophlebitis or deep venous thrombosis, and rheumatoid arthritis17.
Different EDT devices are able to deliver electromagnetic waves at different frequencies. They include Capacitive Resistive Electric Transfer (TECAR) devices. They are used indifferently in the clinical practice.
However, to the best of our knowledge, there are no studies investigating the different effects of high-frequency EDT or low-frequency EDT on postoperative pain and edema reduction. Therefore, the aim of our study is to evaluate whether High-Frequency Treatment (HFT) devices (>2 MHz) have a different impact on reducing postoperative pain and swelling after TKA compared with Low-Frequency Treatment (LFT) devices (<2 MHz) in an inpatient rehabilitation setting.
Patients and Methods
Subjects
For our retrospective observational study, we investigated all patients with TKA who were admitted between October 1st, 2018, and March 30th, 2019, at the Zucchi Clinical Institute “San Francesco” Rehabilitation Department for a period of three-weeks hospitalization for intensive rehabilitation, immediately following the surgical intervention. We conducted our study in compliance with the principles of the Declaration of Helsinki.
Among all patients of both sexes who underwent the inpatient rehabilitation program after TKA, we excluded from our observation patients who had had revision TKA, patients affected by severe cognitive deficit and patients who presented any contraindications to EDT (i.e., presence of pacemaker implant, implantable cardioverter defibrillator or any other cardiac implant, neoplasia, local acute infections, history of epilepsy, pregnancy, thrombophlebitis or deep venous thrombosis, and rheumatoid arthritis).
Treatment
Due to the lack of evidence in literature, about the most effective frequency in EDT, HFT devices (>2 MHz) and LFT devices (< 2 MHz) are equally used in the rehabilitation program of our Institute. Patients receive a standard physiotherapy intervention, but they are usually indifferently assigned to one or the other treatment. Therefore, we observed two groups of patients: those who received HFT (ProNexibus device, LocalCare S.r.l., Bereguardo, PV, Italy) and those who received LFT (Tecar HCR 150 device or Tecar HCR 901 device, Unibell, Calco, LC, Italy) during the hospitalization. The settings used are shown in Table 1. It is necessary only for LFT devices to apply a specific conductive substance on the treated surface. According to the instructions of all devices, the clinicians who provided EDT decided the power setting and the number of sessions for each patient, depending on patients’ conditions. In detail, the warm sensation felt by the patient guided the power setting, while swelling and pain relief guided the number of sessions. Only for HFT devices a maximum number of 5 sessions was suggested by the producer.
The clinicians who provided EDT sessions were not the same as the ones who assessed the patients. The latter were unaware of frequency used and they had no contact with patients during their treatment.
Table 1. Treatment settings and devices.
| High frequencies (>2 MHz) | Low frequencies (< 2 MHz) | |
| Device: ProNexibus | Device: Tecar HCR 901 | Device: Tecar HCR 150 |
| Frequency*: 2 or 4 or 8 Mhz | Frequency: 0.49 MHz | Frequency*: 0.44-0.55 MHz |
| Power range*: 15 W to 110 W | Exit Power*: 200 W or 300 W | Exit Power*: 85 W |
| Treatment duration: 10 minutes | Treatment duration: 10 minutes | |
| *Values depending on patient’s conditions. | ||
Outcomes
All outcomes were assessed in three moments: at baseline, when patients entered the rehabilitation setting (i.e., four days after surgery, T0), in the mid-time of the rehabilitation program (14 days after surgery T1) and before hospital discharge (24 days after surgery T2).
Primary Outcome Measures
– Limb circumferences were used to evaluate postoperative edema. The measurement was performed according to the Guidelines for the assessment of lymphoedema of the limbs of the Italian Society for vascular Investigation19. It was carried out in seven points: middle-foot (halfway from the heel to the tip of the first toe), 0 (ankle), 1 (inferior third of leg), 2 (superior third of leg) and 3 (distal part of the knee), 4 (proximal part of the knee) and 5 (thigh).
– We used Numerical Rating Scale (NRS) to assess pain intensity4. It consists of 11 degrees ranging from 0 (no pain) to 10 (the worst imaginable pain).
Secondary Outcome Measures
We measured secondary outcomes as a result of the expected pain and swelling reduction.
– To evaluate knee mobility, degrees of passive knee flexion were measured using a universal goniometer4.
– The Timed Up and Go (TUG) test appears to be a responsive measure of function also directly following joint replacement arthroplasty4. The tester measures the time the patient takes to rise from a chair, to walk at normal speed for 3 meters, to return to the chair and to sit down again.
– The plan for pain management used in the Institute during the inpatient rehabilitation period follows the SIAARTI (Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva) Recommendations for Postoperative pain treatment20.
To evaluate the rate of use of pharmacological pain therapy, we classified it in this way for each patient:
– Level 1: use of acetaminophen alone or in combination with codeine
– Level 2: use of NSAIDs or COXIBs for a brief period of time (3-7 days) +/- acetaminophen
– Level 3: use of opiates or use of NSAIDs / COXIBs for a long period of time (more than 7 days) +/- acetaminophen.
Each patient was classified as belonging to one of these levels at the end of the rehabilitation recovery (T2).
Statistical Analysis
Kolmogorov-Smirnov test was used to test continuous variables in each group for normal distribution.
Linear mixed model analyses for repeated measures (p = 0.05) were made of each of the outcome measure to evaluate changes over time and between groups. The outcome measures were entered as dependent variables, time and group as fixed effects. The crossover effect of time and group was entered as an interaction term.
Significant effects of the time were found. Thus, separately for the two treatment groups, post-hoc analyses were carried out to evaluate pairwise differences in the changes of the outcome measures (using Bonferroni adjusted alpha levels of .0167).
Because of its ordinal nature, the level of use of pharmacological pain therapy of the two groups was compared using the Mann-Whitney U test.
Results
The patients assessed for eligibility were 83. Among them, 16 presented one or more exclusion criteria, therefore 67 patients were included in the observation. Five patients discontinued the investigation at T1, due to anticipated hospital discharge. Figure 1 shows the study flow chart.
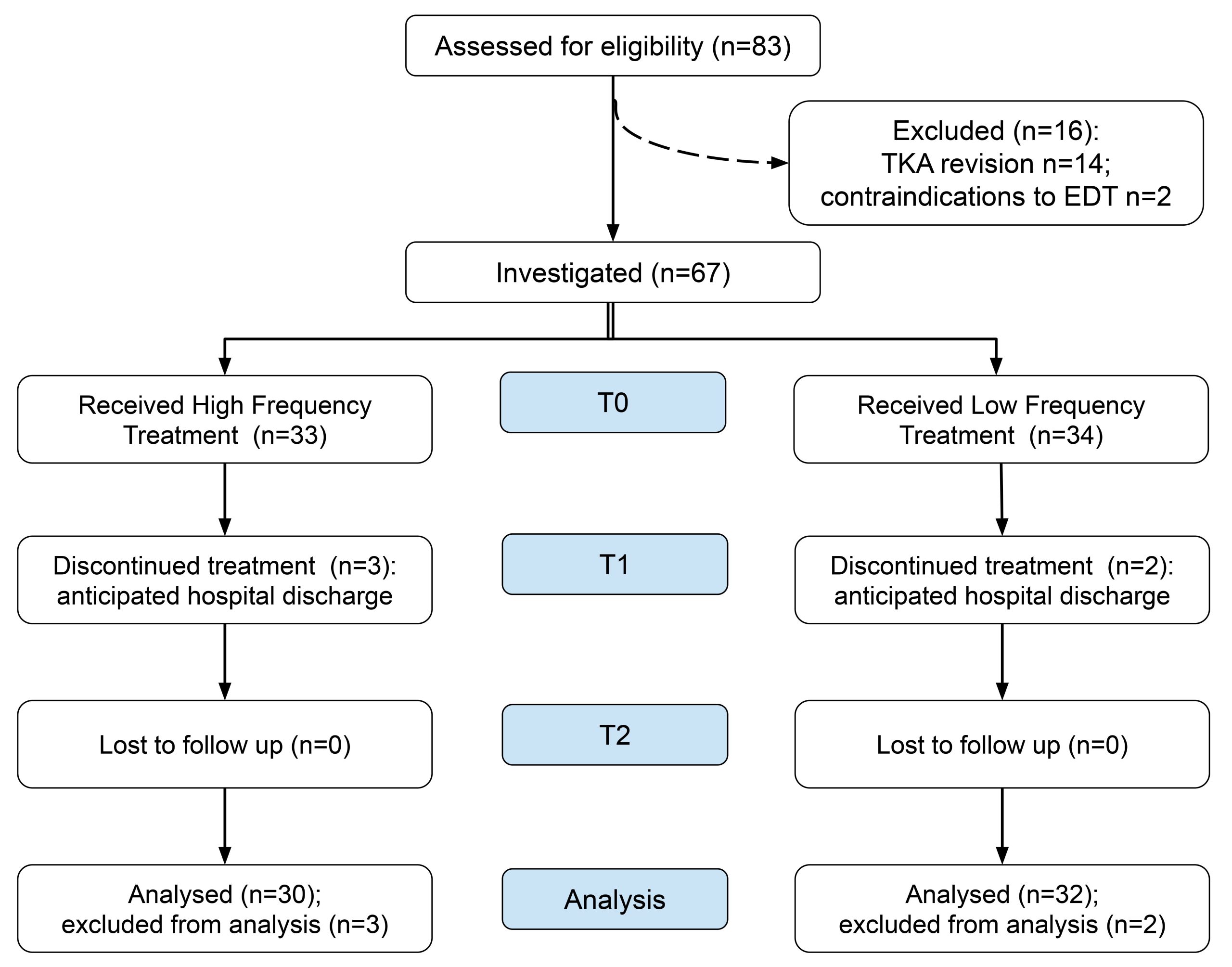
62 patients were investigated, 14 males and 48 females (age range: 50-86 years). 33 subjects were evaluated among those receiving HFT and 34 subjects for LFT.
At T0, subjects of the two groups did not differ for demographic or anthropometric parameters. Patients’ baseline characteristics are shown in Table 2.
Table 2. Subjects’ baseline characteristics (N=62).
| High-frequency treatment (N=30) | Low-frequency treatment (N=32) | p-value | |
| Age (years)a | 69 (8.3) | 72 (7.3) | 0.126 |
| Sex (male/female) | 7/23 | 7/25 | |
| Body Mass Index (kg/m2)a | 30.5 (4.8) | 30.0 (5.1) | 0.470 |
| aMean values (standard deviation). | |||
No adverse events were observed in either group and both HFT and LFT were well tolerated by all patients.
Considering the pharmacotherapy effect as cumulative, we evaluated it at the end of the rehabilitation recovery, categorizing patients in increasing levels of use as we previously showed. As a result, at T2 participants of the two groups did not differ in terms of level of pharmacotherapy used (Mann-Whitney U test).
As reported in Table 3, changes over time within and between groups (HFT vs. LFT) did not show statistically significant group effect on NRS scores (p-value = 0.332), degrees of knee flexion (p-value = 0.973), and TUG results (p-value = 0.620). In the same way, neither the crossover effect of time and group (the interaction effect) resulted statistically significant for NRS scores (p-value = 0.517), degrees of knee flexion (p-value = 0.624), and TUG results (p-value = 0.706). A significant time effect (p-value < 0.001) was found for all outcomes.
Table 3. Changes over time within and between groups for NRS scores, degrees of knee flexion and TUG results (N=62).
| Group | T0* | T1* | T2* | p-value
time effect |
p-value
group effect |
p-value
interaction effect |
|
| NRS
(0-10) |
High Frequency | 6.03 (0.7) | 4.6
(0.7) |
3.23 (0.7) | <0.001 | 0.332 | 0.517 |
| Low Frequency | 6.31 (1.4) | 5.16 (2.1) | 3.34
(0) |
||||
| Degrees of knee flexion | High Frequency | 58.83 (3.5) | 90.17
(0) |
101
(0) |
<0.001 | 0.973 | 0.624 |
| Low Frequency | 57.34 (0) | 91.09 (21.2) | 101.4 (0) | ||||
| TUG (sec) | High Frequency | 36.15 (16.3) | 24.05 (13.4) | 16.1 (7.5) | <0.001 | 0.620 | 0.706 |
| Low Frequency | 37.30 (19.2) | 22.85 (11.1) | 15.2
(4) |
||||
| Mean values (standard deviation). *p<0.05. | |||||||
| NRS, Numeric Rating Scale; TUG, Timed Up and Go test. | |||||||
Table 4 reports the changes over time within and between groups for all the limb circumferences measured. A significant effect of time was found for all outcomes. None of the outcomes showed group effect.
Our results report progressive reduction of pain measured by NRS scale, progressive reduction of limb circumferences, progressive improvement in knee flexion degrees and progressive better performance at the TUG test for both groups throughout the rehabilitation recovery.
As we stated, according to the instructions, pain and swelling reduction guided the clinicians in determining the number of sessions needed for each patient, and only for HFT devices producers indicated 5 as the maximum deliverable sessions. We observed that the average number of sessions for patients in HFT group was 4.9 (range 4-5), while the average number of sessions for patients in LFT group was 10.7 (range 5-16).
Table 4. Changes over time within and between groups for limb circumferences.
| Group | T0* | T1* | T2* | p-value
time effect |
p-value
group effect |
p-value
interaction effect |
|
| middle foot | High Frequency | 22.75 (1.1) | 22.5 (0.7) | 22.25 (1.1) | <0.001 | 0.104 | 0.411 |
| Low Frequency | 22.25 (0.4) | 22 (0) | 21.75 (0.3) | ||||
| 0 – ankle | High Frequency | 27.25 (0.3) | 26.75 (1.8) | 24.75 (0.3) | <0.001 | 0.072 | 0.612 |
| Low Frequency | 24.5 (3.5) | 26 (1.4) | 25.85 (1.2) | ||||
| 1 – inferior third of leg | High Frequency | 30.5 (3.5) | 22.9 (0.8) | 21.5 (0.7) | <0.001 | 0.183 | 0.157
|
| Low Frequency | 26.5 (2.1) | 24.75 (1.8) | 25.75 (2.5) | ||||
| 2 – superior third of leg | High Frequency | 36 (1.4) | 31.75 (3.2) | 30.5 (2.1) | <0.001 | 0.272 | 0.158 |
| Low Frequency | 34 (1.4) | 34 (1.4) | 35.5 (1.4) | ||||
| 3 – distal part of the knee | High Frequency | 39.5 (0.7) | 35.75 (1.1) | 37.25 (0.3) | <0.001 | 0.409 | 0.768 |
| Low Frequency | 40.25 (3.9) | 37.5 (0.7) | 39.75 (1.8) | ||||
| 4 – proximal part of the knee | High Frequency | 48.5 (2.1) | 46.75 (1.8) | 45.65 (1.2) | <0.001 | 0.389 | 0.346 |
| Low Frequency | 48.75 (4.6) | 44.25 (1.8) | 44.25 (3.2) | ||||
| 5 – thigh | High Frequency | 55.75 (1.1) | 49.5 (2.1) | 49.5 (2.1) | <0.001 | 0.496 | 0.630 |
| Low Frequency | 49.5 (5) | 47.75 (2.5) | 47.25 (3.2) | ||||
| Mean values (standard deviation). *p<0.05. | |||||||
Discussion
Drug-free interventions to reduce postoperative pain and swelling after TKA are consistent with the principles of enhanced recovery after surgery and there is increased interest in such nonpharmacological treatments. In fact, pain and swelling are major complaints in most patients after TKA and the risk of persistent postsurgical pain onset is higher when acute pain is not effectively treated21.
Recent literature has highlighted the role of EDT in swelling and pain reduction. Focusing the application of EDT on edema reduction, Cau et al14 studied severely obese subjects with bilateral lower limb lymphedema undergoing EDT in addition to a multidisciplinary rehabilitation program. Compared to the control group, a significant volume reduction in the whole limb and in the thigh was observed after 6 EDT sessions. As secondary outcomes, the TUG and VAS for pain showed improvement in both groups. Although lymphedema has different etiological origins compared to post-surgical edema, our observational study shows similar conclusions on similar outcomes.
In a recent double-blind RCT evaluating the efficacy of diathermy in the postoperative phase of TKA, Garcìa-Marìn et al17 found that the addition of EDT to physiotherapy obtained better results for knee pain than physiotherapy alone. In fact, therapy group (EDT at 0.84 MHz + physiotherapy) showed better results in VAS and WOMAC scales than both the placebo group (turned-off device + physiotherapy) and the control group (physiotherapy only).
Assuming EDT documented efficacy after TKA5,17 and in many other conditions22-24, our study aimed at evaluating possible differences between High frequency EDT treatment and Low frequency EDT treatment in terms of postoperative pain relief and improvement of knee function. Therefore, we focused our observation during inpatient rehabilitation period. Considering that both groups received the same rehabilitation program, and no difference has been shown on the use of pharmacological therapy, our results report for both groups a progressive reduction of pain measured by NRS scale and a progressive reduction of limb circumferences throughout the rehabilitation recovery. These results are accompanied by a consequent improvement in knee flexion, as the reduction of pain and swelling reduces the risk of knee stiffness. In the same way, we observed progressive better performance at the TUG test for both groups, as functional recovery parameters.
These results suggest that there is no difference in terms of EDT efficacy on pain and edema reduction using different frequencies. However, some further considerations can be made.
As we already stated, it is necessary for the LFT devices to apply a specific conductive substance to the treated surface. Therefore, this makes the use of LFT devices in the area closely adjacent to the surgical wound impossible. On the contrary, HFT devices are directly applied to the treated area, and they can also be used above the plaster. Moreover, according to the instructions, the number of sessions was depending on patients’ conditions, but only for HFT devices producers suggested a maximum number of 5 sessions. Our results showed that the average number of sessions for patients in LFT group was 10.7, ranging from a minimum of 5 sessions to a maximum for 16. On the other side the average number of sessions for patients in HFT group was 4.9 (range 4-5).
Although both treatments have shown comparable results, these aspects (the use of conductive substance and different number of sessions) should be considered when tailoring a postoperative rehabilitation program, preferring one or the other treatment according to patient’s needs and hospital’s assets.
Limitations
Our study has some limitations. First of all, it is not a randomized controlled trial, but a retrospective observational study, without any intervention that would change the ordinary protocol used in the Institute. This is the reason why we lack a sham group. However, using EDT, a sham treatment is generally difficult to recruit because of the comfortable deep heating sensation normally felt by patients during the treatment17. Another possible limitation is that pharmacotherapy, notwithstanding our study categorization, may exert a confounding effect that could have affected EDT contribution to pain and swelling reduction. Further studies will be necessary to understand the respective impact of EDT, physiotherapy and drug therapy on the reduction of pain and swelling during the rehabilitation following TKA.
Conclusions
As literature shows, EDT is a valid non-pharmacological option for pain relieving and edema reduction in postoperative inpatient rehabilitation after TKA. It is safe and well tolerated by patients, and presents few contraindications limiting its use. In our study, no difference of effect by HFT vs. LFT devices has been observed, although HFT devices show some characteristics that could make them preferable in some rehabilitation settings, such as a lower number of sessions needed (maximum of 5) and the absence of conductive substance to use.
Conflicts of Interest
The authors declare no conflicts of interest.
Informed Consent
Not applicable due to the retrospective nature of the study.
Ethics Approval
The study was performed in compliance with the principles of the Declaration of Helsinki.
Data Availability
Data are available on medical records of Zucchi Clinical Institute.
References
- Leitner L, Türk S, Heidinger M, Stöckl B, Posch F, Maurer-Ertl W, Leithner A, Sadoghi P. Trends and Economic Impact of Hip and Knee Arthroplasty in Central Europe: Findings from the Austrian National Database. Sci Rep 2018; 8: 4707.
- Lübbeke A, Silman AJ, Barea C, Prieto-Alhambra D, Carr AJ. Mapping existing hip and knee replacement registries in Europe. Health Policy 2018; 122: 548-557.
- Klug A, Gramlich Y, Rudert M, Drees P, Hoffmann R, Weißenberger M, Kutzner KP. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg Sports Traumatol Arthrosc 2021; 29: 3287-3298.
- Mont MA, Banerjee S, Jauregui JJ, Cherian JJ, Kapadia BH. What Outcome Metrics Do the Various Knee Rating Systems for Assessment of Outcomes Following Total Knee Arthroplasty Measure? A Systematic Review of Literature. Surg Technol Int 2015; 26: 269-274.
- Wylde V, Dennis J, Gooberman-Hill R, Beswick AD. Effectiveness of postdischarge interventions for reducing the severity of chronic pain after total knee replacement: systematic review of randomised controlled trials. BMJ Open 2018; 8: e020368.
- Usichenko TI., Edinger H, Witstruck T, Pavlovic D, Zach M, Lange J, Gizhko V, Wendt M, Koch B, Lehmann C. Millimetre wave therapy for pain relief after total knee arthroplasty: a randomised controlled trial. Eur J Pain 2008; 12: 617-623.
- Yue C, Zhang X, Zhu Y, Jia Y, Wang H, Liu Y. Systematic Review of Three Electrical Stimulation Techniques for Rehabilitation After Total Knee Arthroplasty. J Arthroplasty 2018; 33: 2330-2337.
- Henderson KG, Wallis JA, Snowdon DA. Active physiotherapy interventions following total knee arthroplasty in the hospital and inpatient rehabilitation settings: a systematic review and meta-analysis. Physiotherapy 2018; 104: 25-35.
- Tashiro Y, Hasegawa S, Yokota Y, Nishiguchi S, Fukutani N, Shirooka H, Tasaka S, Matsushita T, Matsubara K, Nakayama Y, Sonoda T, Tsuboyama T, Aoyama T. Effect of capacitive and resistive electric transfer on haemoglobin saturation and tissue temperature. Int J Hyperthermia 2017; 33: 696-702.
- Clijsen R, Leoni D, Schneebeli A, Cescon C, Soldini E, Li L, Barbero M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A Pilot Feasibility Study on Healthy Subjects. J Altern Complement Med 2020; 26: 147-153.
- Duñabeitia I, Arrieta H, Torres-Unda J, Gil J, Santos-Concejero J, Gil SM, Irazusta J, Bidaurrazaga-Letona I. Effects of a capacitive-resistive electric transfer therapy on physiological and biomechanical parameters in recreational runners: A randomized controlled crossover trial. Phys Ther Sport 2018; 32: 227-234.
- Beltrame R, Ronconi G, Ferrara PE, Salgovic L, Vercelli S, Solaro C, Ferriero G. Capacitive and resistive electric transfer therapy in rehabilitation: a systematic review. Int J Rehabil Res 2020; 43: 291-298.
- Notarnicola A, Maccagnano G, Gallone MF, Covelli I, Tafuri S, Moretti B. Short term efficacy of capacitive-resistive diathermy therapy in patients with low back pain: a prospective randomized controlled trial. J Biol Regul Homeost Agents 2017; 31: 509-515.
- Cau N, Cimolin V, Aspesi V, Galli M, Postiglione F, Todisco A, Tacchini E, Darno D, Capodaglio P. Preliminary evidence of effectiveness of TECAR in lymphedema. Lymphology 2019; 52: 35-43.
- Coccetta CA, Sale P, Ferrara PE, Specchia A, Maccauro G, Ferriero G, Ronconi G. Effects of capacitive and resistive electric transfer therapy in patients with knee osteoarthritis: a randomized controlled trial. Int J Rehabil Res 2019; 42: 106-111.
- Kumaran B, Watson T. Treatment using 448kHz capacitive resistive monopolar radiofrequency improves pain and function in patients with osteoarthritis of the knee joint: a randomised controlled trial. Physiotherapy 2019; 105: 98-107.
- García-Marín M, Rodríguez-Almagro D, Castellote-Caballero Y, Achalandabaso-Ochoa A, Lomas-Vega R, Ibáñez-Vera AJ. Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. J Clin Med 2021; 10: 1611.
- Adie S, Naylor JM, Harris IA. Cryotherapy after total knee arthroplasty a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 2010. 25: 709-715.
- Antignani PL, Benedetti-Valentini F, Aluigi L, Baroncelli TA, Camporese G, Failla G, Martinelli O, Palasciano GC, Pulli R, Rispoli P, Amato A, Amitrano M, Dorigo W, Gossetti B, Irace L, Laurito A, Magnoni F, Minucci S, Pedrini L, Righi D, Verlato F; Italian Society for Vascular Investigation. Diagnosis of vascular diseases. Ultrasound investigations-guidelines. Int Angiol 2012; 31: 1-77.
- Savoia G, Alampi D, Amantea B, Ambrosio F, Arcioni R, Berti M, Bettelli G, Bertini L, Bosco M, Casati A, Castelletti I, Carassiti M, Coluzzi F, Costantini A, Danelli G, Evangelista M, Finco G, Gatti A, Gravino E, Launo C, Loreto M, Mediati R, Mokini Z, Mondello E, Palermo S, Paoletti F, Paolicchi A, Petrini F, Piacevoli Q, Rizza A, Sabato AF, Santangelo E, Troglio E, Mattia C; SIAARTI Study Group. Postoperative pain treatment SIAARTI Recommendations 2010. Short version. Minerva Anestesiol 2010; 76: 657-667.
- Veal FC, Bereznicki LR, Thompson AJ, Peterson GM, Orlikowski C, Barkin R. Subacute pain as a predictor of long-term pain following orthopedic surgery: an Australian Prospective 12 Month Observational Cohort Study. Medicine 2015; 94: 1.
- Casagrande J, Zoia C, Clerici G, Uccella L, Tabano A. Single level anterior cervical discectomy and fusion for cervical disc herniation in a professional soccer player. J Sports Med Phys Fitness 2016; 56: 754-757.
- Yokota Y, Sonoda T, Tashiro Y, Suzuki Y, Kajiwara Y, Zeidan H, Nakayama Y, Kawagoe M, Shimoura K, Tatsumi M, Nakai K, Nishida Y, Bito T, Yoshimi S, Aoyama T. Effect of Capacitive and Resistive electric transfer on changes in muscle flexibility and lumbopelvic alignment after fatiguing exercise. J Phys Ther Sci 2018; 30: 719-725.
- Bito T, Tashiro Y, Suzuki Y, Kajiwara Y, Zeidan H, Kawagoe M, Sonoda T, Nakayama Y, Yokota Y, Shimoura K, Tatsumi M, Nakai K, Nishida Y, Yoshimi S, Tsuboyama T, Aoyama T. Acute effects of capacitive and resistive electric transfer (CRet) on the Achilles tendon. Electromagn Biol Med 2019; 38: 48-54.
To cite this article
The use of endogenous diathermy therapy for pain and swelling after total knee arthroplasty: comparison of high frequency vs. low frequency treatment
JOINTS 2023;
1: e639
DOI: 10.26355/joints_20236_639
Publication History
Submission date: 09 Mar 2023
Revised on: 03 May 2022
Accepted on: 05 Jun 2023
Published online: 21 Jun 2023