JOINTS 2024;
2: e931
DOI: 10.26355/joints_20244_931
Comprehensive management of lower limb tendinopathies in athletes: advances and challenges
Topic: Sport Medicine
Category: Narrative Review
Abstract
Lower limb tendinopathies are prevalent and challenging conditions in athletes, demanding a complex approach to diagnosis, management, and prevention. This review synthesizes current knowledge and advances in the field, offering insights into effective strategies for treating and preventing these injuries. The complex interaction of biomechanical, physiological, and external factors in tendinopathies necessitates individualized treatment plans. Recent advancements in diagnostic imaging and therapeutic interventions have significantly enhanced our understanding and management of these conditions. The review explores various treatment modalities, including pharmacological interventions, physical therapies, and surgical options. The efficacy of hyaluronic acid, cellular therapies, and platelet-rich plasma (PRP) in promoting tendon healing is examined alongside the potential of newer approaches like stem cell therapy. The role of surgery, primarily as a last resort, is discussed, highlighting advancements from open techniques to minimally invasive procedures. Return to Play (RTP) strategies are critical in managing athletic tendinopathies. A structured RTP process, balancing complete recovery and minimizing deconditioning, is essential. This review emphasizes the importance of gradual and controlled progression in the RTP phase to prevent re-injury. Additionally, the role of prevention through education, biomechanical assessments, and training load management is underscored. This review calls for a collaborative approach in sports medicine, integrating research, clinical practice, and athlete experiences. Such collaboration is vital for developing innovative solutions, ensuring athletes can continue their pursuits with minimal disruption from tendinopathies.
Introduction
Tendinopathies encompass a range of tendon disorders characterized by pain, swelling, and impaired functional performance1. This term refers to various changes in damaged and diseased tendons, leading to pain and reduced function, including tears, inflammatory enthesitis, or chronic degeneration2.
Historically, tendon pain accompanied by decreased function was termed tendinitis3. Tendinitis implies an inflammatory response accompanying tendon injury. However, histopathology studies4 comparing healthy and injured (symptomatic) tendons reveal that these injured tendons are often in a degenerative state with few or no inflammatory cells present.
These disorders are prevalent in the general population but are particularly concerning among athletes, especially in disciplines involving running, jumping, and repetitive strain on specific tendon groups5. Tendons must withstand, store, and then deliver substantial force for daily activities. In sports-related activities, where loading repetition and speed are drastically increased, the mechanical force exerted on the tendon is significantly amplified, demanding greater tendon capacity6. The prevalence of these injuries varies based on the sport, training intensity, athlete’s age, and other biomechanical factors.
Even in cases of healing, symptom resolution and return to full activity can take more than two years, with the progression often being slow and inconsistent. The pathology of tendinopathies is not yet fully understood; there are still many questions about the role of the inflammatory component in healing, the degenerative aspect, neovascularization, and the influence of intrinsic factors on the healing process7. As sports medicine evolves, understanding the complexities of tendinopathies becomes crucial, not just for treatment but also for prevention.
Therefore, this study aims to comprehensively analyze the etiology, diagnosis, treatment, and prevention strategies for tendinopathies, focusing on enhancing the understanding and management of these complex conditions in athletes.
Pathophysiology
Understanding tendinopathies’ pathophysiology is crucial for its diagnosis and treatment8. A deeper insight into etiopathology is essential to develop targeted treatments. The tendon, though seemingly simple, has a complex microarchitecture pivotal to its function and, when disrupted, contributes to the manifestation of tendinopathies9.
Tendons consist of densely packed collagen fibers, primarily type I collagen, which impart tensile strength1. These fibers, along with specialized proteins like fibrillin and proteoglycans, form the extracellular matrix (ECM) and are organized into fascicles. These fascicles are surrounded by the endotenon, and the entire tendon is encased in a sheath known as the epitenon10. Tenocytes, the primary cellular component within this collagenous matrix, are responsible for maintaining and repairing the tendon matrix. In a normal tendon, the extracellular matrix is dense, with predominantly parallel-aligned collagen fibers, primarily type I collagen, interspersed with proteoglycans, glycosaminoglycans, and glycoproteins, including small leucine-rich proteoglycans2.
Tendons adapt to varying loads, changing in response to the stresses they endure. This process, known as “mechanotransduction”, involves tenocytes transforming mechanical stimuli into cellular responses, promoting the transcription of new proteins and collagen, thus altering the tendon’s structure11,12. While tendons can adapt to increased loads by enhancing collagen density and mechanical properties, excessive or repetitive loading can surpass their adaptive capacity13. Neural mechanisms also play a role in this regulation, with evidence of increased corticospinal excitability but simultaneous inhibition of other cortical areas in tendinopathies14. The complexity of these peripheral and central mechanisms during tendon mechanical stimulation contributes to the unclear pathophysiology of tendinopathy.
Several factors can contribute to the onset of a tendon disorder scenario in athletes (Table 1)15. These risk factors can trigger the development of tendinopathy. The concept of preclinical disease, where clinical symptoms are not yet apparent, is supported by genetic susceptibility data. Studies15,16 have identified single-nucleotide variants in genes like COL5A1, MMP3, TIMP2, and TNC. Human tissue analyses of asymptomatic tendons show dysregulation of the extracellular matrix, immune responses, and stromal responses. Failure of normal homeostatic responses can lead to early tendinopathy, characterized by immune cell influx, stromal cell dysfunction, apoptosis, oxidative stress, and matrix dysfunction. Dysregulation of repair mechanisms, such as auto-amplificatory loops and matrix-stromal-immune crosstalk dysfunction, leads to established or chronic tendinopathy, marked by poor function, pain, and reduced load capacity.
Table 1. Risk factors of tendinopathies in athletes.
| External risk factors | Internal risk factors |
| Training mistakes (excessive loading, few resting times, wrong technique) | Wrong posture |
| Wrong play or training field | Dysmorphisms |
| Wrong play or training equipment | Muscles imbalances |
| Psychological factors | Age, sex, BMI |
| Wrong food supplements | Genetic factors |
| Previous unresolved injuries | Metabolic factors: diabetes mellitus, hyperlipidemia |
| Medications: fluoroquinolones, hormone replacement therapy | Altered neuromuscular control |
BMI: body mass index.
In tendinopathy, tenocytes decrease in volume, elongate, have an increased nucleus-to-cytoplasm ratio, and produce less ECM but with an increase in type III collagen density16. Histological examination of patient biopsy samples has shown2,16,17 intratendinous collagen degeneration, fiber disorientation, glycosaminoglycan accumulation between thinning fibrils, and inflammatory cell infiltrates. Neovascularization and neoinnervation are also frequently observed in diseased tendons2. The tendon continuum model, introduced in 2009, classifies tendinopathy based on structural changes in tendons: normal tendon, reactive tendon, tendon disrepair, and degenerative tendinopathy17. A tendon may simultaneously exhibit multiple phases or progress through the continuum, with histological changes depleting its mechanical properties17,18.
Even if pain could be considered a personal experience, influenced by biological, psychological, and social factors, nociceptive input seems to play a key role in the development of tendon pain19. However, the exact source of nociception in tendinopathies remains debated, and multifactorial causes seem to be present20,21. The increase in intra-tendinous pressure that could occur in tendons after a mechanical overload can activate mechanical nociceptors located in the peri tendinous connective tissue and be considered a source of nociception22. On the other hand, tenocytes could release neurotrophic and neuroinflammatory mediators, which can cause nerve sprouting and nerve fiber ingrowth into the tendon. This will expose those fibers to cytokines and neuropeptides, such as substance P, found in tendinopathic tendons. These nerve fibers can become sensitized, leading to pain even with minimal stimuli23.
Collagen fiber disorganization and increased ground substance can alter the tendon’s biomechanical properties, leading to microtears under stress and exacerbating pain24,25. Reduced blood flow or impaired vascular supply can cause localized hypoxia, contributing to pain and further tissue degeneration. These peripheral nociceptive inputs could sensitize the nervous system both at the peripheral, spinal, and cortical levels26, and these sensitization mechanisms could play a role in the transition from an acute to a chronic pain state27. The lack of a strong association between nociceptive input and tendon pain suggests that a combination of these and other cortical factors likely determines tendon pain28. Consequently, the use of repetitive transcranial magnetic stimulation techniques has been theorized for muscle disorders, including tendinopathies23.
Epidemiology
The incidence of lower limb tendinopathy is reported to be 10.52 per 1,000 person-years, surpassing even the incidence of osteoarthritis29. In the lower extremity, the most common tendinopathies occur at the heel (plantar fascia and Achilles tendon), the greater trochanter (gluteal insertional complex), the knee (patellar tendon), and the ankle (tibialis posterior tendon)30. Reports suggest that 1-2% of adults experience lower extremity tendinopathy during their lifetime31.
The patellar tendon, connecting the patella to the tibia, is crucial for knee extension and is particularly stressed during jumping activities. Patellar tendinopathy, often termed “jumper’s knee”, is characterized by progressive activity-related anterior knee pain and focal patellar tenderness32. This condition can significantly impact an athlete’s career, sometimes leading to its premature end. Approximately 20% of athletes experience patellar tendinopathy at some point, particularly in sports like volleyball (24.8%), basketball (20.8%), and soccer (6.1%). It is more prevalent in men and those over 18 years33. In soccer, it often resolves within six days in about 60% of cases but has a high recurrence rate34. Key risk factors include gender, sport type, training hours, and age35. High prevalence is noted36 in sports involving jumping, with the greatest risk during landing, particularly from a horizontal jump. Associations have also been found37 between patellar tendinopathy and decreased hamstring and quadriceps flexibility, as well as low ankle dorsiflexion38.
Achilles tendinopathy, affecting the largest and strongest tendon in the human body, is common among runners, with up to 10% experiencing it at some point39,40. It can be divided into midportion and insertional types. The incidence rate in the adult population is 2.35 per 1,00041, with a lifetime prevalence of 23.9% in athletes compared to 5.9% in the general population42. In elite soccer, it accounts for about 30% of all tendinopathies in outfield players and 16% in goalkeepers, with an average of one case per season and a median absence of 9 days43. Risk factors include prior lower limb tendinopathy or fracture, certain medications, moderate alcohol use, and specific biomechanical patterns (Figure 1)42. Genetic markers also contribute to the risk profile, though the associations are currently ambiguous. According to a recent article by Della Villa et al44, non-contact (83%) or indirect contact (17%) injuries are the most common causes of Achilles tendon rupture in professional football, a very serious and potentially career-ending injury.
While the patellar and Achilles tendons are frequently discussed, other tendons in the lower limb are also susceptible to tendinopathies. Quadriceps tendinopathy, affecting the tendon connecting the quadriceps to the patella, is common in basketball and volleyball players45. Peroneal tendon disorders, involving the tendons running behind the outer ankle bone, are significant causes of posterolateral ankle symptoms in active individuals following both acute lateral ankle sprains (subluxation or dislocation) as well as chronic ankle instability (hypertrophic tendinopathy and tears or ruptures)46. In particular, peroneal tendon dislocation is most prevalent in sports that require cutting movements, including soccer and basketball47. In athletes with acute dislocation, the surgical approach with a combination of groove deepening and superior peroneal retinaculum repair is the recommended choice for appropriate treatment48. Posterior tibialis tendinopathy, affecting the tendon along the inner side of the ankle and foot, is common in runners, particularly those with flat feet. It is often associated with adult-acquired flatfoot deformity and can result from various causes, including trauma, anatomical, mechanical, inflammatory, and ischemic factors49.
Figure 1. Biomechanical analysis of running pattern of an athlete: overpronation with gait line alterations.

Diagnosis
A recent consensus study50 has established “tendinopathy” as the preferred terminology for persistent tendon pain and loss of function related to mechanical loading. The diagnosis of tendinopathy primarily hinges on clinical symptoms and patient history, particularly focusing on activity-provoked localized tendon pain and stiffness.
Imaging modalities, while not essential for diagnosis, are frequently used to confirm the condition and assess its severity, especially in elite athletes50. Ultrasound and magnetic resonance imaging (MRI) are the cornerstone imaging techniques for diagnosing tendinopathies (Figure 2). These modalities have been extensively studied and discussed in academic and clinical settings. However, it is important to note that tendon structural disorganization does not always correlate directly with clinical symptoms. Imaging findings can sometimes create a confusing clinical picture, as they reveal the presence and extent of structural changes within the tendon but require careful interpretation in the context of pain characteristics and aggravating loads20.
Figure 2. Comparison between ultrasound (US) and magnetic resonance imaging (MRI) of an Achilles tendinopathy.

Ultrasound is a non-invasive, cost-effective modality offering real-time dynamic imaging. Common ultrasonographic findings20 associated with tendinopathy include tendon thickening, hypoechoic regions, loss of collagen organization or alignment, and possible neovascularization. However, its effectiveness can be limited by operator dependency and difficulty visualizing deep-seated tendons.
Axial-strain sonoelastography is an innovative ultrasonography technique for measuring tissue hardness. It involves manual axial compression of tissue using a hand-held ultrasound transducer to generate tissue strains (deformations)51. Its role in imaging Achilles tendinopathy has grown recently, offering additional insights into the biomechanical properties of the tendon compared to conventional ultrasound.
MRI provides detailed images of the tendon structure and surrounding tissues, including any associated inflammation. However, it is more expensive and less accessible than ultrasound. Additionally, as a static imaging modality, MRI cannot assess dynamic changes during movement.
Emerging techniques in imaging, particularly the integration of artificial intelligence (AI), are revolutionizing the diagnosis of tendinopathies52. Convolutional neural networks (CNNs), a class of deep learning algorithms, are particularly promising in this regard. CNNs are adept at processing and analyzing complex visual data, making them ideal for interpreting medical images such as ultrasounds and MRIs53.
Conservative Treatments
Multiple rehabilitation strategies are recommended for patients with tendinopathy. These approaches, though diverse in mechanisms, aim to reduce symptoms, particularly pain, promote tendon healing, and enhance patient function. Effective tendinopathy management necessitates a multifaceted approach, tailored to the specific tendon involved, the condition’s severity, and the athlete’s needs (Table 2). A patient-centered and personalized approach is essential in every case. Educating patients about the disease process, acknowledging any previous unsuccessful treatments if relevant, understanding the potentially prolonged time frames for management, and addressing any fear mechanisms associated with starting treatment are crucial steps.
Table 2. Proposed treatment algorithm for tendinopathies management in athletes.
| Step 0 | Patient education and load management | |
| Step 1 | Exercise therapy | – Progressive individualized strengthening program that incorporates the current evidence-based principles of load and exercise progression
– At least 12 weeks |
| ± ESWT | – Emax 0.15-0.20 mJ/mmq
– Adjustment of therapy protocols according to athletic needs of performance and RTP, – Rest in the following 24-48 hours |
|
| ± Nutraceutical | – Collagen or gelatin + vitamin C 1 hour before exercise
– Higher protein intake (2-2.5 g protein/kg BM-1/D-1), 20-30 g/meal, 4-6 meals/day, 30-40 g casein protein before sleep – supplementation with free-form essential amino acids (plateaus at 15-18 g), adequate hydration (5-7 ml/kg BM), daily ingestion of 3 fish oils (4 g/day), Vitamin D and creatine (20 g/D-1 x 5 d, 5 g/d) |
|
| If failure, progress: | ||
| Step 2 | Percutaneous electrolysis | – Absence of Doppler positivity and a tendon not too thick
– Intensity (mA): 1-6 mA, – Periodization: 0-7-14-14. |
| Injections | – HA
– Stem cells – PRP – Sclerosant agent |
|
| If failure, progress: | ||
| Step 3 | Surgery | |
ESWT: extracorporeal shock wave therapy; HA: hyaluronic acid; PRP: platelet-rich plasma; RTP: return to play.
Central to tendinopathy management, exercise therapy aims to restore tendon strength and function. Tendon-loading programs are currently the most effective conservative treatment approach. Exercise therapy, focusing largely on resistance exercise, often eccentric actions, encourages load tolerance, leading to structural and functional improvements at the musculotendinous unit54,55. It is supported by the highest level of scientific evidence and is always recommended as the initial therapy step56. Exercise therapy has been shown to promote collagen fiber cross-linking, facilitate tendon remodeling, and cause an upregulation of insulin-like growth factor (IGF), which aids in cellular proliferation, matrix remodeling, and tendon fiber reorganization and healing1. Eccentric exercise has been a preferred treatment for chronic tendinopathy, especially in patellar and Achilles tendinopathies, for over 30 years57,58, since it has been beneficial both at microscopical59 and macroscopical level60 (Figure 3). Subsequently, different methodological options to treat chronic tendinopathy, using different exercise modalities, have been developed, such as heavy slow resistance61, isoinertial62, and isometric63: this latter, in particular, shows an important analgesic effect. However, to date, there is no strong evidence to support the use of a single type of exercise when treating tendinopathies43,64. Load and speed parameters might influence tendon healing more than the exercise type itself, and sports withdrawal should not be a dogma65,66. In clinical trials66,67 on the use of exercise for tendinopathy, the length of the interventions tends to be about 12 weeks. Several randomized clinical trials (RCT)67 did not report any disadvantage due to continued sports activity, so it is possible to let the patient train with pain but with particular attention to its eventual worsening. The pain monitoring model helps patients understand the permissible amount of pain during and after exercise68. Moreover, interventions including additional weight, training once daily, and a higher volume of resistance exercises are effective69.
The treatment of tendinopathy often involves various modalities, typically used in conjunction with exercise. Some adjuvant therapies are combined or used sequentially. Many adjuvants to exercise lack strong evidence for or against their use, but their safety is established with varying effectiveness.
Figure 3. Examples of eccentric exercises in the rehabilitation pathway of a professional football player with patellar tendinopathy.

Physical Therapy
Nowadays, one of the most effective biophysical therapies in tendinopathy management is Extracorporeal Shock Waves Therapy (ESWT)70. Shock waves (SW) are acoustic waves (mechanical stimulations) that, when applied to living tissues, do not cause damage but rather can induce some therapeutic biological reactions through the phenomenon known as mechanotransduction71,72. SW has been shown71 to modify cellular membrane permeability, increase nitric oxide production, dilute neurotransmitters (like Substance P), reduce small unmyelinated nerve fibers, and generally have an antinociceptive effect. Additionally, they can enhance the synthesis of growth factors such as Vascular Endothelial Growth Factor (VEGF) and Bone Morphogenetic Proteins (BMPs), promote angiogenesis, vasculogenesis, and lymphangiogenesis, as well as stem cell proliferation, migration, “homing” and differentiation72. From a general point of view, it is reasonable to say that SW can induce a tissue-specific regenerative effect by stimulating the self-healing ability of our body by modulating some inflammatory pathways73,74. SW also plays a significant role in regulating macrophage functions by promoting the shift towards the M2 (or pro-resolving) phenotype71. Cellular components of innate immunity have a key role in maintaining tendon homeostasis, both in healthy tendons and in tendinopathies. Moreover, they can be recruited following a tendon injury and may regulate tendon healing and remodeling75. The mechanical stimulus provided by ESWT might initiate tendon regeneration by promoting pro-inflammatory and catabolic processes associated with removing damaged matrix constituents as a prelude to restoration of homeostasis76. From a clinical point of view, ESWT has been proven effective in both patellar and Achilles tendinopathy, with most studies76-79 reporting positive effects, a success rate ranging from 65% to 91%, and a low rate of complications. Nevertheless, ESWT should always be accompanied by a comprehensive supervised exercise program: Zhang et al80 showed that patients with Achilles tendinopathy who had greater sports activity levels had better therapeutic responses than nonsports-active patients after a 5-year follow-up. While its actual regenerative effects take weeks, it can optimize the rehabilitation pathway and accelerate RTP already in the short and medium term.
Another interesting instrumental technique that is gaining scientific attention nowadays is percutaneous electrolysis81. Percutaneous needle electrolysis (PNE) is a minimally invasive technique in which a galvanic is conveyed through an acupuncture needle into the non-homogeneous target area identified under direct ultrasound visualization82. The main technical difficulty is the ultrasound visualization of the needle, but it is a safe procedure83. It produces an analgesic and fibrinolytic effect and a local inflammatory process in musculoskeletal soft tissues that stimulates the repair of the affected tissue, such as tendon84, as shown by its effect in patellar tendinopathy85 or plantar fascitis86. Galvanic current induces an inflammatory process mediated by inflammasome activation by promoting tissue regeneration and remodeling processes87. The histological and functional evidence observed in an animal model of muscle lesion demonstrates that the application of PNE during muscle regeneration induces a decrease in pro-inflammatory mediators (TNF-α and IL-1β) and an increase in the expression of anti-inflammatory proteins (PPAR-γ) and VEGF88,89. Further RCTs are needed to widely recommend its use, even if it has interesting and promising applications.
Injection Therapies
Various injection therapies have been tested for tendinopathies, yielding different results. Hyaluronic acid (HA) has been extensively tested81 for tendinopathies. It plays a viscoelastic and biological role. HA is a significant component of tendon structure, being abundantly present in the extracellular space. It exhibits a lubricating action and has shown solid evidence of beneficial effects on tenocytes, including increased viability, metabolic activity, and expression of type I collagen, along with a reduction in apoptosis. Its low cost and high safety profile are additional important aspects. HA also integrates well with physical therapies. In the extracellular space of tendons, HA facilitates tendon gliding, reduces adhesions, improves tendon architectural organization, and limits inflammation90. Scientific literature reports showed results in terms of functional recovery, pain reduction, and improved mobility. In their prospective multicentric clinical trial, Frizziero et al91 demonstrated that one weekly injection for three weeks under ultrasound guidance of HA (500-730 kDa) induced prompt improvement in pain and function in mid-portion Achilles and patellar tendinopathies, lasting up to 90 days post-treatment. Furthermore, HA has shown promising effects when combined with ESWT92. Fogli et al93 found that ultrasound-guided HA (500-730 kDa) peri-tendinous injections significantly relieved pain and reduced tendon thickness and neo-vascularization in ultrasound evaluations. HA proved effective in both low to medium molecular weight formulations (3 injections)94 and in a single injection of high molecular weight (2,700 KD)95 in improving various outcomes of different tendinopathies. However, there is no consensus in the literature regarding the optimal timing for starting infiltrative therapy with hyaluronic acid.
The use of cellular therapy, including progenitor cells, stem cells, and autologous tenocytes, in treating tendon disorders is on the rise2. Stem cells have shown significant potential for tissue repair and regeneration, making them a promising therapeutic option in sports medicine. Orthobiologics harness a high biological potential, leveraging a rich array of growth factors, cytokines, chemokines, and mesenchymal stem cells (MSCs). Sources include synovial membrane, bone marrow, and adipose tissue96, with the latter becoming preferred due to minimal harvest morbidity, high cell yield, and a large volume of available tissue. Adipose tissue is abundant, easy to harvest, has minimal morbidity, and offers a higher MSC frequency volume rate compared to other sources10. This has led to increasing interest in using adipose tissue-derived MSCs for treating various pathologies, including tendinopathies97. However, many studies97,98 in this area have only provided level IV evidence of the efficacy of cellular therapy. The RCT by Usuelli et al98 compared a single injection of intratendinous adipose-derived stromal vascular fraction (SVF) with a single injection of platelet-rich plasma (PRP) for treating 44 patients with Achilles tendinopathy. The authors followed the patients for up to six months, evaluating them with clinical scores and imaging analyses. No complications were observed in either group. Both treatments offered clinical improvement from baseline to six months, with SVF providing faster clinical improvement: higher scores at 15 and 30 days compared to PRP (p<0.05), but no differences at longer follow-up and in imaging analyses. The injective treatment with SVF has been recently evaluated75 for patellar tendinopathy, showing promising results in terms of clinical improvements and imaging findings. The balance between pro- and anti-inflammatory cells (M1/M2) significantly impacts the resolution of the inflammatory process and thus tendon healing; inadequate or unregulated inflammation resolution, with a persistent prevalence of pro-inflammatory type 1 macrophages, could lead to chronic inflammation, fibrosis, and tendon degeneration (hyaline, mucoid, fatty)75. A potential strategy involves controlling the immune system by increasing macrophage recruitment into the lesion to induce the polarization from inflammatory M1 to regenerative M2 phenotypes as well as activate endogenous stem cell regenerative ability99,100. Thus, the role of Peripheral Blood Mononuclear Cells (PB-MNCs) in treating tendinopathies has been hypothesized100. In a case series by Caravaggio et al101, authors presented 27 cases of partial Achilles tendon injury, 6 women and 21 men, ranging in age from 23 to 71 years, treated with a single outpatient injection of PBMNCs. All subjects were evaluated at baseline and clinically after 2 months, using the American Orthopedic Foot & Ankle Society – AOFAS scale, and radiologically (MRI examination). Moreover, a clinical reassessment using the AOFAS scale was performed at six months. No adverse effects were recorded in any patients. Functional and radiological signs of tendon healing processes were detected as early as two months after the procedure, and the AOFAS scale rose from an initial average value of 37.5 to 85.4. Although the small sample size (n=27), this preliminary result suggests that autologous PB-MNCs can represent an innovative and safe therapeutic alternative to surgical options for Achilles tendinopathy and tendon partial injuries.
Sclerosants, aimed at obliterating neovessels and nerve ingrowth, are believed to be sources of pain in tendinopathies102. Lower limb tendinopathies that can benefit from the use of sclerosing injections are those that are chronic and symptomatic, with tendon tissue alterations at ultrasound or MRI examination, with hypervascularization supported by neovessels penetrating the patellar tendon from the posterior wall and the Achilles tendon from the ventral wall103-106. These vessels guide a nerve branch into the tendon that runs near the vessels themselves, responsible for the pain104,106. The drug used for extra tendinous infiltrations into neovessels is polidocanol (Ethoxysclerol), an anesthetic with sclerosing properties for vessels. An ultrasound-guided injection is performed with 2 mL of polidocanol (5 mg/mL or 10 mg/mL), causing the immediate closure of the neovessels, with the disappearance of intratendinous hyperperfusion and subsequent ischemia and destruction of the nerve fiber adjacent to the neovessels104,107. Two weeks after the treatment, sports activities can be resumed. If the result is not satisfactory, the treatment can be repeated after 3-4 weeks for 3-5 times. Usually, there are no significant side effects107. However, in the treatment of these tendon pathologies, to minimize recurrences and with the aim of a more accurate evaluation of results, it is advisable to consider predisposing factors, attempting to eliminate modifiable ones.
Several studies108 have investigated the use of PRP for managing tendinopathy at different anatomical sites, yielding varying results. PRP is a preparation of autologous blood centrifuged to contain a high concentration of platelets, with or without leukocytes. Platelet degranulation releases several factors, including TGFβ, PDGF, bFGF, VEGF, IGF1, and EGF, all involved in different phases of tendon healing. PRP demonstrated109 the therapeutic potential to promote cell proliferation and differentiation, regulate angiogenesis, increase extracellular matrix synthesis, and modulate inflammation in degenerative tendons. However, controversy exists within the literature regarding the clinical use of PRP for tendinopathy. Indeed, although PRP continues to be used as a treatment option, studies109,110 providing high-level evidence have not confirmed its significant efficacy in tendinopathy treatment. A systematic review and meta-analysis110 showed the efficacy of both leukocyte rich-PRP and the leukocyte poor-PRP treatment for lateral epicondylitis, while less evidence supports the use in patellar tendinopathy. Another systematic review109 analyzing outcomes following PRP injection for patellar tendinopathy found inconsistent results in comparative studies demonstrating the superiority of PRP over placebo or other treatments, although PRP showed promise in non-comparative studies. The evidence for the efficacy of PRP in treating Achilles tendinopathy in athletes is mixed. The lower level-of-evidence case series and retrospective studies110,111 demonstrate a clear pattern of positive results.
Nutraceuticals
Finally, several dietary strategies and nutritional supplements have been studied112 for the comprehensive management of tendinopathies, including collagen peptides, Vitamin C, and omega-3 fatty acids. However, in comparison to muscle, the science of nutritional interventions to improve soft tissue function remains in its infancy112. Indeed, the physiology of tendons and ligaments is different from muscle, given that soft tissues have limited blood flow and are dependent on nutrient delivery through bulk fluid flow112.
However, some evidence comes from in vitro and in vivo studies113-115. Paxton et al113 reported that the amino acid proline with vitamin C improved collagen synthesis, and Shawn et al114 demonstrated that 15 g of gelatin (ingested with 50 mg of Vitamin C) one hour before exercise increased blood markers related to collagen synthesis in the athletic population.
Although more research is required, evidence suggests that the ingestion of gelatin is a promising nutritional intervention to improve both the function of connective tissues and speed the recovery from musculoskeletal injuries. Interestingly, Praet et al115 concluded that oral supplementation of specific collagen peptides might accelerate the clinical benefits of a structured calf-strengthening and return-to-running program in Achilles tendinopathy patients.
In line with this context, monitoring the overall energy intake of athletes during the injury phase and recovery (RTP process) is also of fundamental importance116,117.
Finally, it is important to consider nutrition advice on what to avoid during injury. Beyond excessive energy intake, alcohol intake should also be discouraged. Alcohol intake after training and competition reduces rates of myofibrillar protein synthesis even when co-ingested with protein118. Moreover, the suppression of the anabolic response in skeletal muscle will impair recovery and adaptation to the rehabilitation program of the injured players116.
Surgery
Surgery for tendinopathy theoretically aims to promote regenerative healing by triggering a reparative response in the matrix environment119. Surgical procedures for tendinopathy typically involve excising degenerative tendon tissue, removing adhesions around the tendon, decompressing the tendon, and/or performing multiple longitudinal tenotomies120. These procedures have evolved from open techniques to minimally invasive approaches using arthroscopy or percutaneous incisions under image guidance2,121.
In about 90% of cases, conservative treatment for patellar tendinopathy is effective. When it fails, surgery becomes a viable option. Tenotomy with excision of tendinopathic tissue is the most common surgical procedure for patellar tendinopathy, yielding results similar to those of eccentric loading alone122. Patellar tendon rupture, a severe and potentially career-ending injury, occurs when the patellar tendon tears or detaches completely from the kneecap or shinbone. Fluoroquinolone antibiotics have been implicated123 in some cases of patellar tendon rupture. This injury often results from a strong eccentric contraction of the quadriceps muscle under load while the knee is partially flexed, typically causing a rupture in the central region of the tendon124. Athletes often report a “popping” sensation at the time of injury, followed by an inability to straighten the knee. Depending on the severity, classified by the Popkin-Golman classification, treatment can be surgical or conservative125.
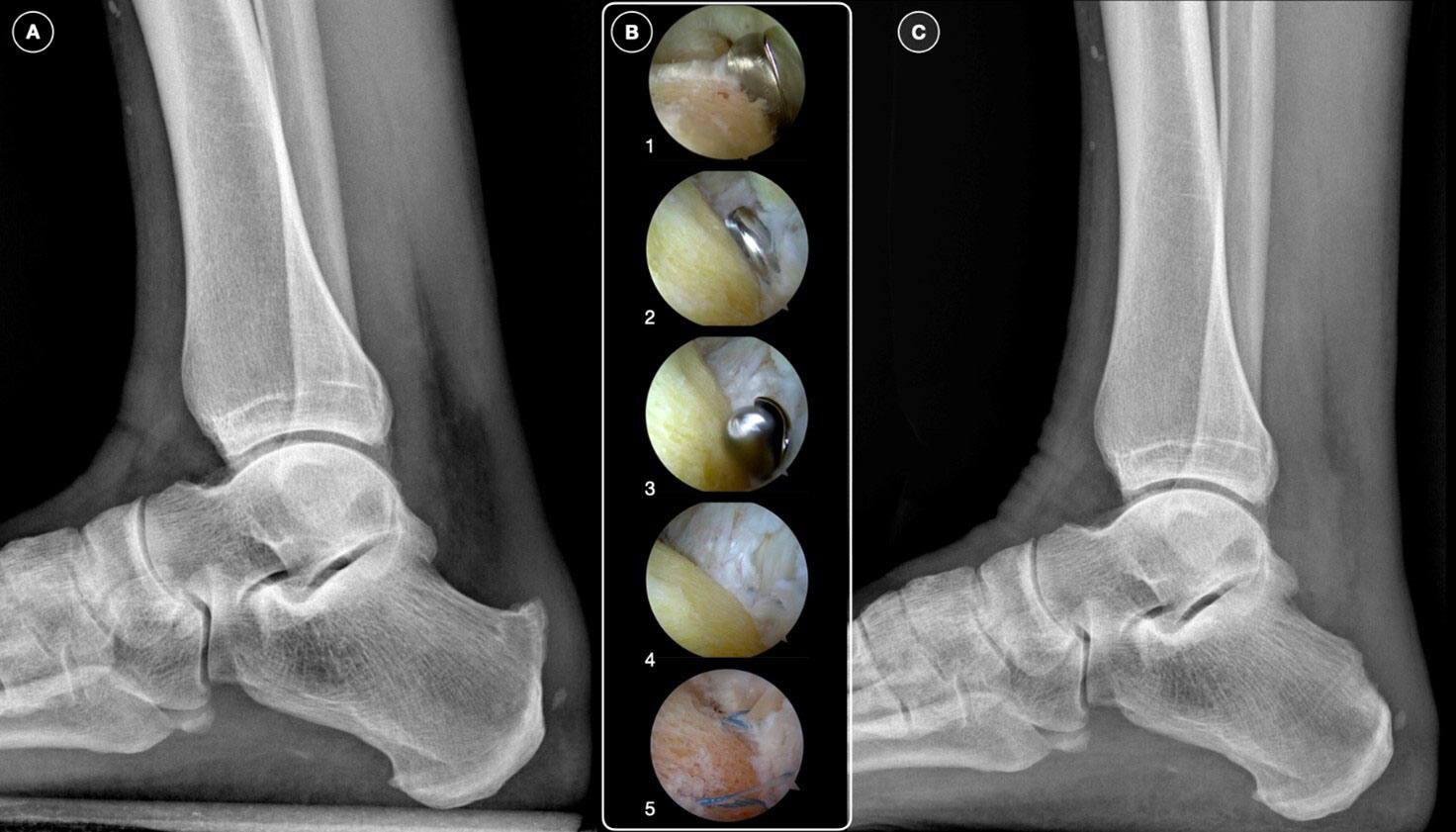
For Achilles tendinopathy patients unresponsive to conservative treatments after 6-12 months, surgical intervention is considered. In cases of midportion Achilles tendinopathy, a pedicled soleus transplant can facilitate a quicker return to running without pain and reduce the need for surgical revisions. Insertional tendinopathy, often associated with Haglund’s disease, involves degeneration of the Achilles tendon fibers at the calcaneal insertion126. Surgical treatment includes debridement of the Achilles insertional region and resection of the calcaneal exostosis (calcaneoplasty), which can be performed either openly or endoscopically, the latter being associated with fewer complications and quicker recovery127,128 (Figure 4). In high-demanding athletes, reinforcing the Achilles tendon may be recommended to minimize the risk of AT detachment and expedite healing and recovery, although return-to-play (RTP) times can vary significantly. A complete tear of the Achilles tendon, characterized by sudden sharp pain, is a medical emergency with increasing incidence129. Treatment options include conservative management and surgical repair130. There is no consensus on which is the best treatment for Achilles tendon ruptures, and their management is still controversial. A recent systematic review131 found that surgical treatment had a lower re-rupture rate compared to conservative treatment, but conservative management had fewer complications, except for re-ruptures. Open repair and minimally invasive surgery had similar re-rupture rates, but open repair had fewer complications, including lower rates of sural nerve injuries. No significant differences were found in re-rupture rates or complications between early and late rehabilitation across different treatment modalities. For acute major trauma in the midportion, minimally invasive surgery with a transversal cut and high-resistance Fiber Wire is preferred, while chronic proximal/distal degenerative lesions are better treated with open surgical procedures involving transfer or transposition. It is important to underline that in the case of tenorrhaphy of the Achilles tendon, the use of eccentric exercises in the rehabilitation program must be very cautious132. Furthermore, during Achilles tendon post-surgical rehabilitation, the progression of the intensity of the proposed exercises should be carefully monitored via MRI132.
Figure 4. A, A preoperative lateral ankle radiograph showing a Haglund deformity. B, Calcaneal exostectomy performed by endoscopy: the posterior calcaneal tubercle is resected with a burr (1-4); in selected cases, a reinforcement with anchors of the Achilles insertional area is possible (5). C, Postoperative radiograph showing resulted in calcaneal exostectomy.
Return to Play and Prevention Strategies
Returning to sport after tendinopathy is a challenging process that requires balancing complete recovery with minimizing deconditioning. A structured approach, often under the guidance of a sports medicine professional, is crucial for optimizing outcomes133.
RTP is a process, not a single event134. Typically, physiotherapists may follow a process where they first identify the key performance indicators of their sport, determine the physical attributes that correspond to these indicators, and then distribute the development of these capacities over the allocated timeframe135. Effective training plans are based on a theoretical or biological understanding of how we move and adapt to exercise stimuli. This understanding, coupled with knowledge of how these stimuli are best sequenced, allows one stimulus and subsequent adaptation to potentiate the next. Thus, reverse or backward engineering, when appropriately converged with plans devised around nutrition, conditioning, technical, and tactical training, likely gives athletes the best chance of attaining their performance goals (Figure 5)136,137.
Figure 5. Return to play the player’s profile.

Irrespective of the treatment path, a critical question remains: how can we most efficiently return patients to sports with a low risk of reinjury or other injuries? Inadequate rehabilitation and premature return to sport are risks that might be minimized with appropriate guidance during the return-to-sport phase138. Reinjury rates of Achilles tendinopathy in soccer players range from 27% to 44%. Notably, reinjury is more common following short recovery periods and in those who received no assistance with the return-to-sport phase, compared with those who followed a standardized progression program that gradually increased loading139. Therefore, during the return-to-sport phase, it is important to have a gradual and controlled progression that allows the athlete sufficient time to recover and gives the therapist time to evaluate symptoms. Since athletes may not experience symptoms from the Achilles tendon during sports participation, they may be tempted to return prematurely. Evaluating symptoms such as stiffness, pain, and swelling after training, especially the following day, can assist in determining appropriate increases in training intensity or volume68.
In the literature140, the resumption of activities such as running and jumping is generally recommended when symptoms have subsided. Often, studies140 include an intervention for a minimum of 12 weeks, after which return to sport is allowed. However, resting from sporting activities during the early phase of treatment may not be necessary141. Various factors need to be considered when planning the return to sport after lower-limb tendinopathy (Table 3)142. The guiding principle of the return-to-sport program is to progressively increase the demand on the tendon by controlling the intensity, duration, and frequency of tendon loading142.
Table 3. Return to play factors to be considered after tendinopathies142.
| Pain and symptoms (pain-monitoring model) |
| Tendon healing and recovery |
| Impairments and risk factors: recovery of strength, ROM and function |
| Load management |
| Sport-specific demands |
ROM: range of motion.
Silbernagel et al142 described an RTP model consisting of a progressive rehabilitation pathway:
- Educate the athlete about the injury and explain the pain-monitoring model.
- Initiate the program when the athlete can perform activities of daily living with pain no higher than 2/10. Determine and classify specific activities as light, medium, or high level based on pain rating during and after the activity and the athlete’s perceived Achilles tendon exertion. Light-level activity can be performed daily. After a medium-level activity, two days of recovery are needed, during which the athlete cannot perform activities of the same or higher level. High-level activities require three days of recovery after medium- and/or high-level activities.
- When the athlete improves (i.e., the pain level and the perceived exertion level decrease), a new activity classification is performed, usually revisited every three weeks. A previous medium-level activity might then become light, and a new activity can be added to the high list.
A recent systematic review143 identified some criteria for RTP after Achilles tendinopathies (Table 4), while the same process after Achilles tendon rupture is even more complicated (Table 5).
Table 4. Criteria for return to play after Achilles tendinopathy143.
| Pain | – No pain during sports activities
– No severe pain – Pain 5/10 on VAS – No increase in pain – Minimal residual tenderness – Minimal pain (2/10 on NRS) with daily activities |
| Functional recovery | – Capable of completing a full practice
– Able to walk comfortably at 4 mph for 10 miles – Regaining full function – Ability to perform and control sport-specific skills |
| Recovery of muscular strength | – Recovery of full strength
– Power – No calf muscle weakness – No muscle imbalance – Strenght equal to the contralateral limb – LSI ≥90% |
| Recovery of ROM | – Recovery of full ROM
– No altered mobility of foot/ankle – ROM equal to contralateral limb |
| Level of endurance | – Recovery of full endurance
– Completing 3 series of 20 one-legged heel lifts on the stairs without increased pain – Adequate endurance |
| Medical advice | – Completed rehabilitation program
– Gradual stepwise training program – Gradual return-to-sport specific function – Physical examination – Specific investigations – Demands of the specific sport |
| Psychosocial factors | – Individual goals
– Mental aspects – Confidence |
| Anatomical/physiological properties of the musculotendinous complex | – Proprioceptive control
– Healing and recovery of tendon tissue – Rates and magnitudes of Achilles tendon load |
VAS: visual analogic scale; NRS: numeric rating scale; LSI: limb symmetry index; ROM: range of motion.
Table 5. Return to play after Achilles tendon rupture.
| Target area | Test | Measurement | Required target | |
| Swelling | Palpation | Palpation | Absence144 | |
| Pain | Pain during functional activities | VAS | No pain/minimal during and after activity145 | |
| ROM | Full ROM (dorsi/plantarflexion) | Goniometer (Weight-bearing) | >95% LSI144 | |
| Function of injured area | Strength | Standing calf isometric test | 1RM overcome force plate | >3x BW (peak force) |
| Seated calf isometric test | 1RM overcome force plate | >2x BW (peak force) | ||
| Big Toe flexor strength | 1RM dynamometer | >2.7 N/kg146 | ||
| Calf capacity test. Post training session (after main day) | No. repetitions | >25 reps (single leg) BW | ||
| Stability | Star excursion balance test. Post training (after main day) | Centimeters + quality | >95% LSI147 | |
| Plyometrics | Single leg drops jumps (30 cm box) | Force platforms (GCT; RSI; Jump Height) | LSI >20 cm + 95% >LSI | |
| Drop jump (30 cm box) | Force platforms (GCT; RSI; Jump Height) | >30 cm | ||
| SL repeated (3) broad jump | Centimeters + quality | >90% LSI148 | ||
| Run* | Running | Fitness test used by team | Pre-injury level | >95% pre-injury |
| Average Team value/positional Volume/High speed running value | GPS | At least x2.5 match demands149 | ||
| Coding | t-test | Speed-gate | >90% pre-injury level or <10 sec150 | |
| 30 mt sprint test | Speed-gate | >90% pre-injury level | ||
| Sport-specific* | Training volume | At least 2-3 weeks of full training without limitations | ||
| Context-specific | High demands of chaos | Psychological/Observation | ||
*: Only in case of absence from sport >8 weeks. BW: body weight; LSI: limb symmetry index.
Finally, evidence suggests that long-term interventions, including balance training, may be effective in preventing patellar and Achilles tendinopathy. Shoe adaptations, such as shock-absorbing insoles, could have a preventive effect on Achilles tendinopathy. Hormone replacement therapy appears to reduce the risk of structural Achilles tendon changes in active post-menopausal women. No evidence supports the positive effect of stretching exercises. Prophylactic eccentric training and stretching can increase the risk of injury in asymptomatic players with patellar tendon abnormalities. More research is needed on the (multifactorial) etiology, risk factors, and preventive interventions for tendinopathy144.
Conclusions
Tendinopathies of the lower limb, while common among athletes, present significant challenges in terms of diagnosis, management, and prevention. The intricate interplay of biomechanical, physiological, and external factors makes each case unique, necessitating a tailored approach to treatment. Advancements in research, particularly in imaging and therapeutic interventions, have deepened our understanding of these conditions, leading to more effective treatment strategies.
However, the adage “prevention is better than cure” is particularly apt for tendinopathies. As the sports community continues to push the boundaries of human performance, athletes, coaches, and healthcare professionals must prioritize tendon health. Through a combination of education, biomechanical assessments, and careful management of training loads, the incidence and impact of tendinopathies can be significantly reduced.
In the ever-evolving field of sports medicine, collaboration among researchers, clinicians, and athletes is key to developing innovative solutions. This collaborative approach ensures that athletes can pursue their passions with minimal hindrance from tendinopathies, maintaining both their performance and well-being.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Ethics Approval
Not applicable.
Informed Consent
Not applicable.
Funding
None.
Acknowledgments
The authors would like to extend their heartfelt gratitude to all the members of the Sports Medicine Group of SIAGASCOT for their invaluable contributions to this research. Their expertise, dedication, and hard work have been instrumental in the success of this project, and their insights have greatly enriched our study.
*A. Bertelli1, P. Bettinsoli2, M. Bigoni3, G.N. Bisciotti4, L. Boldrini5,6, F. Caravaggio7,8, F. Cortese9, M.C. D’Agostino10, G. De Guttry11, S. Di Antonio12,13, A. Di Martino14, F. Di Pietto15, M. Freschi16, D. Lama17, L. Mancin18, C. Mazzola19, G. Nanni20, A. Pantalone21, E. Pelosin22,23, M. Perasso24, L. Perticarini25, A. Quaglia17, G. Thiebat26, L. Vergani24, G. Zanon27
1Institute of Sports Medicine, Turin, Italy
2Clinic Institute “Sant’Anna”, Brescia, Italy
3School of Medicine and Surgery, Milan-Bicocca University, Milan, Italy
4Kinemove Rehabilitation Centers, Pontremoli – La Spezia, Italy
5Isokinetic Medical Group, FIFA Medical Centre of Excellence, Milan, Italy
6MilanLab Research Department, AC Milan, Milan, Italy
7Foot and Ankle Surgery Center, “Casa di Cura Città di Parma”, Parma, Italy
8Associazione Ex Alumni “G. Pisani” Cuneo, Italy
9Ospedale Santa Maria del Carmine, Rovereto, Italy
10Shock Waves Therapy and Research Center, Rehabilitation Department, Humanitas Clinical and Research Center, IRCCS, Rozzano, Milan, Italy
11San Camillo Hospital, Forte dei Marmi, Lucca, Italy
12Department of Health Science and Technology, Center for Pain and Neuroplasticity (CNAP), SMI, School
of Medicine, Aalborg University, Aalborg, Denmark
13Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal Child Health,
Genoa, Italy
14IRCCS Istituto Ortopedico Rizzoli, Clinica Ortopedica e Traumatologica 2, Applied and Translational
Research Center, Bologna, Italy
15Dipartimento di Diagnostica per Immagini, Pineta Grande Hospital – Castel Volturno, Caserta, Italy
16Juventus Football Club, Turin, Italy
17FC Internazionale Milano, Milan, Italy
18Department of Biomedical Science, University of Padova, Padova, Italy
19Ospedali Galliera, Genoa, Italy
20Isokinetic Medical Group, Bologna, Italy
21Clinic of Orthopaedics and Traumatology, Department of Medicine and Aging Sciences, “G. d’Annunzio”
University of Chieti-Pescara, Chieti, Italy
22IRCCS Policlinico San Martino, Genoa, Italy
23Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal Child Health (DINOGMI), University of Genoa, Genoa, Italy
24Genoa Cricket and Football Club, Genoa, Italy
25Fondazione Poliambulanza Istituti Ospedalieri, Sezione di Traumatologia dello Sport e Chirurgia Protesica Robotica, Brescia, Italy
26IRCCS Istituto Clinico San Siro, Milan, Italy
27Casa di Cura “I Cedri”, Fara Novarese, Novara, Italy
Authors’ Contributions
S.P. played a pivotal role in conceptualizing the study design, was actively involved in the data collection process, and contributed significantly to the data analysis. S.P. also participated in the drafting and critical revision of the manuscript, ensuring the accuracy and integrity of the work presented. M.G. contributed extensively to the study’s methodology development and was instrumental in the acquisition of data. Additionally, M.G. played a key role in interpreting the data and provided substantial input in the writing and editing of the manuscript. A.C. was deeply involved in the data analysis and interpretation. A.C.’s expertise was crucial in the statistical analysis of the data, and he contributed significantly to the drafting of the manuscript, particularly in the discussion and conclusion sections.
References
- Loiacono C, Palermi S, Massa B, Belviso I, Romano V, Gregorio AD, Sirico F, Sacco AM. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina (Kaunas) 2019; 55: 447.
- Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA. Tendinopathy. Nat Rev Dis Primers 2021; 7: 1.
- Filardo G, Di Matteo B, Kon E, Merli G, Marcacci M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc 2018; 26: 1984-1999.
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 2003; 22: 675-692.
- Mercurio M, Corona K, Galasso O, Cerciello S, Morris BJ, Guerra G, Gasparini G. Soccer players show the highest seasonal groin pain prevalence and the longest time loss from sport among 500 athletes from major team sports. Knee Surg Sports Traumatol Arthrosc 2022; 30: 2149-2157.
- Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech 2004; 37: 865-877.
- Masci L. Is tendinopathy research at a crossroads? Br J Sports Med 2015; 49: 1030-1031.
- Hopkins C, Fu SC, Chua E, Hu X, Rolf C, Mattila VM, Qin L, Yung PS, Chan KM. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol 2016; 4: 9-20.
- Ferretti A, Puddu G, Mariani PP, Neri M. The natural history of jumper’s knee. Patellar or quadriceps tendonitis. Int Orthop 1985; 8: 239-242.
- Di Meglio F, Sacco AM, Belviso I, Romano V, Sirico F, Loiacono C, Palermi S, Pempinello C, Montagnani S, Nurzynska D, Castaldo C. Influence of supplements and drugs used for the treatment of musculoskeletal disorders on adult human tendon-derived stem cells. Muscles Ligaments Tendons J 2020; 10: 376-384.
- Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res 2015; 33: 832-839.
- Wang HN, Huang YC, Ni GX. Mechanotransduction of stem cells for tendon repair. World J Stem Cells 2020; 12: 952.
- Maganaris CN, Narici M V., Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med 2004; 34: 1005-1017.
- Rio E, Kidgell D, Moseley GL, Gaida J, Docking S, Purdam C, Cook J. Tendon neuroplastic training: Changing the way we think about tendon rehabilitation: A narrative review. Br J Sports Med 2016; 50: 209-215.
- Riley G. Tendinopathy–from basic science to treatment. Nat Clin Pract Rheumatol 2008; 4: 82-89.
- Heinemeier KM, Schjerling P, Øhlenschlæger TF, Eismark C, Olsen J, Kjær M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. FASEB J 2018; 32: 4763-4775.
- Cook JL, Rio E, Purdam CR, Docking SI. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med 2016; 50: 1187-1191.
- Luo J, Wang Z, Tang C, Yin Z, Huang J, Ruan D, Fei Y, Wang C, Mo X, Li J, Zhang J, Fang C, Li J, Chen X, Shen W. Animal model for tendinopathy. J Orthop Translat 2023; 42: 43.
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020; 161: 1976-1982.
- Docking SI, Ooi CC, Connell D. Tendinopathy: Is Imaging Telling Us the Entire Story? J Orthop Sports Phys Ther 2015; 45: 842-852.
- Ackermann PW, Alim MA, Pejler G, Peterson M. Tendon pain – what are the mechanisms behind it? Scand J Pain 2022; 23: 14-24.
- Pringels L, Cook JL, Witvrouw E, Burssens A, Vanden Bossche L, Wezenbeek E. Exploring the role of intratendinous pressure in the pathogenesis of tendon pathology: a narrative review and conceptual framework. Br J Sports Med 2023; 57: 1042-1048.
- De Martino E, Seminowicz DA, Schabrun SM, Petrini L, Graven-Nielsen T. High frequency repetitive transcranial magnetic stimulation to the left dorsolateral prefrontal cortex modulates sensorimotor cortex function in the transition to sustained muscle pain. Neuroimage 2019; 186: 93-102.
- Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy – towards translation. Nat Rev Rheumatol 2017; 13: 110-122.
- Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L, Carr AJ. Inflammation activation and resolution in human tendon disease. Sci Transl Med 2015; 7: 311ra173.
- Fernández-De-Las-Peñas C, Navarro-Santana MJ, Cleland JA, Arias-Buría JL, Plaza-Manzano G. Evidence of Bilateral Localized, but Not Widespread, Pressure Pain Hypersensitivity in Patients With Upper Extremity Tendinopathy/Overuse Injury: A Systematic Review and Meta-Analysis. Phys Ther 2021; 101.
- Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010; 6: 599-606.
- Rio E, Moseley L, Purdam C, Samiric T, Kidgell D, Pearce AJ, Jaberzadeh S, Cook J. The pain of tendinopathy: physiological or pathophysiological? Sports Med 2014; 44: 9-23.
- Albers IS, Zwerver J, Diercks RL, Dekker JH, Van den Akker-Scheek I. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC Musculoskelet Disord 2016; 17: 16.
- Riel H, Lindstrøm CF, Rathleff MS, Jensen MB, Olesen JL. Prevalence and incidence rate of lower-extremity tendinopathies in a Danish general practice: a registry-based study. BMC Musculoskelet Disord 2019; 20: 239.
- Linsell L, Dawson J, Zondervan K, Rose P, Randall T, Fitzpatrick R, Carr A. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care. Rheumatology 2006; 45: 215-221.
- Ferretti A, Papandrea P, Conteduca F. Knee injuries in volleyball. Sports Med 1990; 10: 132-138.
- Nutarelli S, Lodi CMT da, Cook JL, Deabate L, Filardo G. Epidemiology of Patellar Tendinopathy in Athletes and the General Population: A Systematic Review and Meta-analysis. Orthop J Sports Med 2023; 11: 23259671231173659. https://pubmed.ncbi.nlm.nih.gov/37347023/
- Hägglund M, Zwerver J, Ekstrand J. Epidemiology of patellar tendinopathy in elite male soccer players. Am J Sports Med 2011; 39: 1906-1911. https://pubmed.ncbi.nlm.nih.gov/21642599/
- Theodorou A, Komnos G, Hantes M. Patellar tendinopathy: an overview of prevalence, risk factors, screening, diagnosis, treatment and prevention. Arch Orthop Trauma Surg 2023; 143: 6695-6705. https://pubmed.ncbi.nlm.nih.gov/37542006/
- Van Der Worp H, De Poel HJ, Diercks RL, Van Den Akker-Scheek I, Zwerver J. Jumper’s knee or lander’s knee? A systematic review of the relation between jump biomechanics and patellar tendinopathy. Int J Sports Med 2014; 35: 714-722.
- Cook JL, Kiss ZS, Khan KM, Purdam CR, Webster KE. Anthropometry, physical performance, and ultrasound patellar tendon abnormality in elite junior basketball players: a cross-sectional study. Br J Sports Med 2004; 38: 206-209.
- Backman LJ, Danielson P. Low range of ankle dorsiflexion predisposes for patellar tendinopathy in junior elite basketball players: a 1-year prospective study. Am J Sports Med 2011; 39: 2626-2633.
- Tarantino D, Palermi S, Sirico F, Balato G, D’Addona A, Corrado B. Achilles tendon pathologies: How to choose the best treatment. Journal of Human Sport and Exercise. 2020, 15: S1300-S1321.
- Tarantino D, Mottola R, Resta G, Gnasso R, Palermi S, Corrado B, Sirico F, Ruosi C, Aicale R. Achilles Tendinopathy Pathogenesis and Management: A Narrative Review. Int J Environ Res Public Health 2023; 20: 6681.
- Lopes AD, Hespanhol LC, Yeung SS, Costa LOP. What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med 2012; 42: 891-905.
- de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med 2011; 45: 1026-1028.
- Zwerver J, Brink M, Cook J. Tendon injuries in football players: FC Barcelona 2021 tendon guide; 2021. Available at: https://www.lasselempainen.fi/wp-content/uploads/2021/11/FC_BARCELONA_TENDON_GUIDE_2021-web.pdf.
- Della Villa F, Buckthorpe M, Tosarelli F, Zago M, Zaffagnini S, Grassi A. Video analysis of Achilles tendon rupture in male professional football (soccer) players: injury mechanisms, patterns and biomechanics. BMJ Open Sport Exerc Med 2022; 8: e001419.
- Lian ØB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med 2005; 33: 561-567.
- van Dijk PA, Miller D, Calder J, DiGiovanni CW, Kennedy JG, Kerkhoffs GM, Kynsburtg A, Havercamp D, Guillo S, Oliva XM, Pearce CJ, Pereira H, Spennacchio P, Stephen JM, van Dijk CN. The ESSKA-AFAS international consensus statement on peroneal tendon pathologies. Knee Surg Sports Traumatol Arthrosc 2018; 26: 3096-3107.
- Arrowsmith SR, Fleming LL, Allman FL. Traumatic dislocations of the peroneal tendons. Am J Sports Med 1983; 11: 142-146.
- van Dijk PAD, Gianakos AL, Kerkhoffs GMMJ, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc 2016; 24: 1155-1164.
- Guelfi M, Pantalone A, Mirapeix RM, Vanni D, Usuelli FG, Guelfi M, Salini V. Anatomy, pathophysiology and classification of posterior tibial tendon dysfunction. Eur Rev Med Pharmacol Sci 2017; 21: 13-19.
- Scott A, Squier K, Alfredson H, Bahr R, Cook JL, Coombes B, de Vos RJ, Fu SN, Grimaldi A, Lewis JS, Maffulli N, Magnusson SP, Malliaras P, Mc Auliffe S, Oei EHG, Purdam CR, Rees JD, Rio EK, Gravare Silbernagel K, Speed C, Weir A, Wolf JM, Akker-Scheek IVD, Vicenzino BT, Zwerver J. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br J Sports Med 2020; 54: 260-262.
- Zhang Q, Cai Y, Hua Y, Shi J, Wang Y, Wang Y. Sonoelastography shows that Achilles tendons with insertional tendinopathy are harder than asymptomatic tendons. Knee Surg Sports Traumatol Arthrosc 2017; 25: 1839-1848.
- Wang L, Wen D, Yin Y, Zhang P, Wen W, Gao J, Jiang Z. Musculoskeletal Ultrasound Image-Based Radiomics for the Diagnosis of Achilles Tendinopathy in Skiers. J Ultrasound Med 2023; 42: 363-371.
- Amendolara A, Pfister D, Settelmayer M, Shah M, Wu V, Donnelly S, Johnston B, Peterson R, Sant D, Kriak J, Bills K. An Overview of Machine Learning Applications in Sports Injury Prediction. Cureus 2023; 15: e46170.
- Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, Oliveira JM, Pereira H, Peretti GM, Spang C, Stephen J, van Bergen CJA, de Girolamo L. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options. J Exp Orthop 2018; 5: 38.
- Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, Oliveira JM, Pereira H, Peretti GM, Romero-Rodriguez D, Spang C, Stephen J, van Bergen CJA, de Girolamo L. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part I: biology, biomechanics, anatomy and an exercise-based approach. J Exp Orthop 2017; 4: 18.
- Challoumas D, Crosbie G, O’Neill S, Pedret C, Millar NL. Effectiveness of Exercise Treatments with or without Adjuncts for Common Lower Limb Tendinopathies: A Living Systematic Review and Network Meta-analysis. Sports Med Open 2023; 9: 71.
- Silbernagel KG, Thomeé R, Thomeé P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain–a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports 2001; 11: 197-206.
- Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med 1998; 26: 360-366.
- Murtaugh B, Ihm JM. Eccentric training for the treatment of tendinopathies. Curr Sports Med Rep 2013; 12: 175-182.
- Breda SJ, Oei EHG, Zwerver J, Visser E, Waarsing E, Krestin GP, de Vos RJ. Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. Br J Sports Med 2021; 55: 501-509.
- Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M, Magnusson SP. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports 2009; 19: 790-802.
- Romero-Rodriguez D, Gual G, Tesch PA. Efficacy of an inertial resistance training paradigm in the treatment of patellar tendinopathy in athletes: a case-series study. Phys Ther Sport 2011; 12: 43-48.
- Rio E, Kidgell D, Purdam C, Gaida J, Moseley GL, Pearce AJ, Cook J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br J Sports Med 2015; 49: 1277-1283.
- de Vos RJ, van der Vlist AC, Zwerver J, Meuffels DE, Smithuis F, van Ingen R, van der Giesen F, Visser E, Balemans A, Pols M, Veen N, den Ouden M, Weir A. Dutch multidisciplinary guideline on Achilles tendinopathy. Br J Sports Med 2021; 55: 1125-1134.
- Saithna A, Gogna R, Baraza N, Modi C, Spencer S. Suppl 3: Eccentric Exercise Protocols for Patella Tendinopathy: Should we Really be Withdrawing Athletes from Sport? A Systematic Review. Open Orthop J 2012; 6: 553-557.
- Malliaras P, Barton CJ, Reeves ND, Langberg H. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med 2013; 43: 267-286.
- Frizziero A, Trainito S, Oliva F, Nicoli Aldini N, Masiero S, Maffulli N. The role of eccentric exercise in sport injuries rehabilitation. Br Med Bull 2014; 110: 47-75.
- Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med 2007; 35: 897-906.
- Pavlova AV, Shim JSC, Moss R, Maclean C, Brandie D, Mitchell L, Greig L, Parkinson E, Alexander L, Tzortziou Brown V, Morrissey D, Cooper K, Swinton PA. Effect of resistance exercise dose components for tendinopathy management: a systematic review with meta-analysis. Br J Sports Med 2023; 57: bjsports-2022-105754.
- Liao C De, Xie GM, Tsauo JY, Chen HC, Liou TH. Efficacy of extracorporeal shock wave therapy for knee tendinopathies and other soft tissue disorders: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2018; 19: 278.
- Sukubo NG, Tibalt E, Respizzi S, Locati M, d’Agostino MC. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int J Surg 2015; 24: 124-130.
- Vulpiani MC, Vetrano M, Savoia V, Di Pangrazio E, Trischitta D, Ferretti A. Jumper’s knee treatment with extracorporeal shock wave therapy: a long-term follow-up observational study. J Sports Med Phys Fitness 2007; 47: 323-328.
- Mani-Babu S, Morrissey D, Waugh C, Screen H, Barton C. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med 2015; 43: 752-761.
- van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J. ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc 2013; 21: 1451-1458.
- Chisari E, Rehak L, Khan WS, Maffulli N. Tendon healing is adversely affected by low-grade inflammation. J Orthop Surg Res 2021; 16: 700.
- Waugh CM, Morrissey D, Jones E, Riley GP, Langberg H, Screen HR. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy. Eur Cell Mater 2015; 29: 268-280.
- Mani-Babu S, Morrissey D, Waugh C, Screen H, Barton C. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med 2015; 43: 752-761.
- Korakakis V, Whiteley R, Tzavara A, Malliaropoulos N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med 2018; 52: 387-407.
- Saggini R, Di Stefano A, Saggini A, Bellomo RG. Clinical application of shock wave therapy in musculoskeletal disorders: Part II related to myofascial and nerve apparatus. J Biol Regul Homeost Agents 2015; 29: 771-785.
- Zhang S, Li H, Yao W, Hua Y, Li Y. Therapeutic Response of Extracorporeal Shock Wave Therapy for Insertional Achilles Tendinopathy Between Sports-Active and Nonsports-Active Patients With 5-Year Follow-up. Orthop J Sports Med 2020; 8: 2325967119898118.
- Asensio-Olea L, Leirós-Rodríguez R, Marqués-Sánchez MP, de Carvalho FO, Maciel LYS. Efficacy of percutaneous electrolysis for the treatment of tendinopathies: A systematic review and meta-analysis. Clin Rehabil 2023; 37: 747-759.
- Varela-Rodríguez S, Sánchez-Sánchez JL, Velasco E, Delicado-Miralles M, Sánchez-González JL. Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial. J Clin Med 2022; 11: 2889.
- Margalef R, Bosque M, Minaya-Muñoz F, Valera-Garrido F, Santafe MM. Safety analysis of percutaneous needle electrolysis: a study of needle composition, morphology, and electrical resistance. Acupunct Med 2021; 39: 471-477.
- Peñin-Franch A, García-Vidal JA, Martínez CM, Escolar-Reina P, Martínez-Ojeda RM, Gómez AI, Bueno JM, Minaya-Muñoz F, Valera-Garrido F, Medina-Mirapeix F, Pelegrín P. Galvanic current activates the NLRP3 inflammasome to promote Type I collagen production in tendon. Elife 2022; 11: e73675.
- Abat F, Sánchez-Sánchez JL, Martín-Nogueras AM, Calvo-Arenillas JI, Yajeya J, Méndez-Sánchez R, Monllau JC, Gelber PE. Randomized controlled trial comparing the effectiveness of the ultrasound-guided galvanic electrolysis technique (USGET) versus conventional electro-physiotherapeutic treatment on patellar tendinopathy. J Exp Orthop 2016; 3: 34.
- Fernández-Rodríguez T, Fernández-Rolle Á, Truyols-Domínguez S, Benítez-Martínez JC, Casaña-Granell J. Prospective Randomized Trial of Electrolysis for Chronic Plantar Heel Pain. Foot Ankle Int 2018; 39: 1039-1046.
- Valera-Garrido F, Minaya-Muñoz F, Sánchez-Ibáñez JM, García-Palencia P, Valderrama-Canales F, Medina-Mirapeix F, Polidori F. Comparison of the acute inflammatory response and proliferation of dry needling and electrolysis percutaneous intratissue (epi) in healthy rat achilles tendons. Br J Sports Med 2013; 47: e2-e2.
- Peñin-Franch A, García-Vidal JA, Martínez CM, Escolar-Reina P, Martínez-Ojeda RM, Gómez AI, Bueno JM, Minaya-Muñoz F, Valera-Garrido F, Medina-Mirapeix F, Pelegrín P. Galvanic current activates the NLRP3 inflammasome to promote Type I collagen production in tendon. Elife 2022; 11: e73675.
- Abat F, Valles SL, Gelber PE, Polidori F, Jorda A, García-Herreros S, Monllau JC, Sanchez-Ibáñez JM. An experimental study of muscular injury repair in a mouse model of notexin-induced lesion with EPI® technique. BMC Sports Sci Med Rehabil 2015; 7: 7.
- Kaux JF, Samson A, Crielaard JM. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J 2015; 5: 264-269.
- Frizziero A, Oliva F, Vittadini F, Vetrano M, Bernetti A, Giordan N, Vulpiani MC, Santilli V, Masiero S, Maffulli N. Efficacy of ultrasound-guided hyaluronic acid injections in achilles and patellar tendinopathies: A prospective multicentric clinical trial. Muscles Ligaments Tendons J 2019; 9: 305-313.
- Pellegrino R, Brindisino F, Barassi G, Sparvieri E, DI Iorio A, de Sire A, Ruosi C. Combined ultrasound guided peritendinous hyaluronic acid (500-730 Kda) injection with extracorporeal shock waves therapy vs. extracorporeal shock waves therapy-only in the treatment of shoulder pain due to rotator cuff tendinopathy. A randomized clinical J Sports Med Phys Fitness 2022; 62: 1211-1218.
- Fogli M, Giordan N, Mazzoni G. Efficacy and safety of hyaluronic acid (500-730kDa) Ultrasound-guided injections on painful tendinopathies: a prospective, open label, clinical study. Muscles Ligaments Tendons J 2017; 7: 388.
- Gervasi M, Barbieri E, Capparucci I, Annibalini G, Sisti D, Amatori S, Carrabs V, Valli G, Donati Zeppa S, Rocchi MBL, Stocchi V, Sestili P. Treatment of Achilles Tendinopathy in Recreational Runners with Peritendinous Hyaluronic Acid Injections: A Viscoelastometric, Functional, and Biochemical Pilot Study. J Clin Med 2021; 10: 1397.
- Kumai T, Muneta T, Tsuchiya A, Shiraishi M, Ishizaki Y, Sugimoto K, Samoto N, Isomoto S, Tanaka Y, Takakura Y. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): a preliminary study. J Orthop Sci 2014; 19: 603-611.
- Palermi S, Gnasso R, Belviso I, Iommazzo I, Vecchiato M, Marchini A, Corsini A, Vittadini F, Demeco A, De Luca M, Tarantino D, Romano V, Sacco A, Sirico F. Stem cell therapy in sports medicine: current applications, challenges and future perspectives. J Basic Clin Physiol Pharmacol 2023; 34: 699-706.
- Itro A, Trotta MC, Miranda R, Paoletta M, De Cicco A, Lepre CC, Tarantino U, D’Amico M, Toro G, Schiavone Panni A. Why Use Adipose-Derived Mesenchymal Stem Cells in Tendinopathic Patients: A Systematic Review. Pharmaceutics 2022; 14: 1151.
- Usuelli FG, Grassi M, Maccario C, Vigano’ M, Lanfranchi L, Alfieri Montrasio U, de Girolamo L. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc 2018; 26: 2000-2010.
- Alshoubaki YK, Nayer B, Das S, Martino MM. Modulation of the Activity of Stem and Progenitor Cells by Immune Cells. Stem Cells Transl Med 2022; 11: 248-258.
- Masoomikarimi M, Salehi M. Modulation of the Immune System Promotes Tissue Regeneration. Mol Biotechnol 2022; 64: 599-610.
- Caravaggio F, Depalmi F, Antonelli M. Treatment of Achilles tendon partial injuries with injection of peripheral blood mononuclear cells (PB-MNCs): a case series. Eur J Transl Myol 2022; 32: 2022.
- Moura JL, Abreu FG, Queirós CM, Pisanu G, Clechet J, Vieira TD, Sonnery-Cottet B. Ultrasound-Guided Electrocoagulation of Neovessels for Chronic Patellar Tendinopathy. Arthrosc Tech 2020; 9: e803-e807.
- Lind B, Öhberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 2006; 14: 1327-1332.
- Aicale R, Bisaccia RD, Oliviero A, Oliva F, Maffulli N. Current pharmacological approaches to the treatment of tendinopathy. Expert Opin Pharmacother 2020; 21: 1467-1477.
- Alfredson H, Öhberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2005; 13: 338-344.
- Alfredson H, Öhberg L. Neovascularisation in chronic painful patellar tendinosis–promising results after sclerosing neovessels outside the tendon challenge the need for surgery. Knee Surg Sports Traumatol Arthrosc 2005; 13: 74-80.
- Alfredson H, Lorentzon R. Sclerosing polidocanol injections of small vessels to treat the chronic painful tendon. Cardiovasc Hematol Agents Med Chem 2007; 5: 97-100.
- Vetrano M, Castorina A, Vulpiani MC, Baldini R, Pavan A, Ferretti A. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med 2013; 41: 795-803.
- Zhou Y, Wang JHC. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. Biomed Res Int 2016; 2016: 9103792.
- Niemiec P, Szyluk K, Jarosz A, Iwanicki T, Balcerzyk A. Effectiveness of Platelet-Rich Plasma for Lateral Epicondylitis: A Systematic Review and Meta-analysis Based on Achievement of Minimal Clinically Important Difference. Orthop J Sports Med 2022; 10: 23259671221086920.
- Sneed D, Wong C. Platelet-rich plasma injections as a treatment for Achilles tendinopathy and plantar fasciitis in athletes. PMR 2023; 15: 1493-1506.
- West DW, Lee-Barthel A, McIntyre T, Shamim B, Lee CA, Baar K. The exercise-induced biochemical milieu enhances collagen content and tensile strength of engineered ligaments. J Physiol 2015; 593: 4665-4675.
- Lis DM, Jordan M, Lipuma T, Smith T, Schaal K, Baar K. Collagen and Vitamin C Supplementation Increases Lower Limb Rate of Force Development. Int J Sport Nutr Exerc Metab 2022; 32: 65-73.
- Shaw G, Lee-Barthel A, Ross MLR, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr 2017; 105: 136-143.
- Praet SFE, Purdam CR, Welvaert M, Vlahovich N, Lovell G, Burke LM, Gaida JE, Manzanero S, Hughes D, Waddington G. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients 2019; 11: 76.
- Tipton KD. Nutritional Support for Exercise-Induced Injuries. Sports Med 2015; 45 Suppl 1: 93-104.
- Close GL, Baar K, Sale C, Bermon S. Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. Int J Sport Nutr Exerc Metab 2019; 29: 189-197.
- Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, Coffey VG. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One 2014; 9: e88384.
- Millar NL, Murrell GAC, Kirwan P. Time to put down the scalpel? The role of surgery in tendinopathy. Br J Sports Med 2020; 54: 441-442.
- Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res 2008; 466: 1539-1554. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2505250/
- Ferretti A, Conteduca F, Camerucci E, Morelli F. Patellar tendinosis: A follow-up study of surgical treatment. J Bone Joint Surg Am 2002; 84: 2179-2185.
- Bahr R, Fossan B, Løken S, Engebretsen L. Surgical treatment compared with eccentric training for patellar tendinopathy (Jumper’s Knee). A randomized, controlled trial. J Bone Joint Surg Am 2006; 88: 1689-1698.
- Morales DR, Slattery J, Pacurariu A, Pinheiro L, McGettigan P, Kurz X. Relative and Absolute Risk of Tendon Rupture with Fluoroquinolone and Concomitant Fluoroquinolone/Corticosteroid Therapy: Population-Based Nested Case-Control Study. Clin Drug Investig 2019; 39: 205-213.
- Figueroa D, Figueroa F, Calvo R. Patellar Tendinopathy: Diagnosis and Treatment. J Am Acad Orthop Surg 2016; 24: e184-e192.
- Golman M, Wright ML, Wong TT, Lynch TS, Ahmad CS, Thomopoulos S, Popkin CA. Rethinking Patellar Tendinopathy and Partial Patellar Tendon Tears: A Novel Classification System. Am J Sports Med 2020; 48: 359-369.
- Jerosch J, Nasef NM. Endoscopic calcaneoplasty–rationale, surgical technique, and early results: a preliminary report. Knee Surg Sports Traumatol Arthrosc 2003; 11: 190-195.
- Van Dijk CN, Van Dyk GE, Scholten PE, Kort NP. Endoscopic calcaneoplasty. Am J Sports Med 2001; 29: 185-189.
- Guelfi M, Vega J. Endoscopic treatment of insertional Achilles tendinopathy: Surgical techniques and indications. Fuss und Sprunggelenk 2021; 19: 86-94.
- Lantto I, Heikkinen J, Flinkkilä T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand J Med Sci Sports 2015; 25: e133-e138.
- Tarantino D, Palermi S, Sirico F, Corrado B. Achilles Tendon Rupture: Mechanisms of Injury, Principles of Rehabilitation and Return to Play. J Funct Morphol Kinesiol 2020; 5: 95.
- Seow D, Islam W, Randall GW, Azam MT, Duenes ML, Hui J, Pearce CJ, Kennedy JG. Lower re-rupture rates but higher complication rates following surgical versus conservative treatment of acute achilles tendon ruptures: a systematic review of overlapping meta-analyses. Knee Surg Sports Traumatol Arthrosc 2023; 31: 3528-3540.
- Bisciotti GN, Bisciotti A, Auci A, Bisciotti A, Eirale C, Corsini A, Volpi P. Achilles Tendon Repair after Tenorraphy Imaging and the Doughnut Metaphor. Int J Environ Res Public Health 2023; 20: 5985.
- Matheson GO, Shultz R, Bido J, Mitten MJ, Meeuwisse WH, Shrier I. Return-to-play decisions: are they the team physician’s responsibility? Clin J Sport Med 2011; 21: 25-30.
- Creighton DW, Shrier I, Shultz R, Meeuwisse WH, Matheson GO. Return-to-play in sport: a decision-based model. Clin J Sport Med 2010; 20: 379-385.
- Wing CE, Turner AN, Bishop CJ. Importance of Strength and Power on Key Performance Indicators in Elite Youth Soccer. J Strength Cond Res 2020. 34: 2006-2014.
- Chia L, Taylor D, Pappas E, Hegedus EJ, Michener LA. Beginning With the End in Mind: Implementing Backward Design to Improve Sports Injury Rehabilitation Practices. J Orthop Sports Phys Ther 2022; 52: 770-776.
- Turner AN, Read P, Maestroni L, Chavda S, Yao X, Papadopoulos K, Virgile A, Spiegelhalter A, Bishop C. Reverse Engineering in Strength and Conditioning: Applications to Agility Training. Strength Cond J 2022; 44: 85-94.
- Silbernagel KG, Crossley KM. A proposed return-to-sport program for patients with midportion achilles tendinopathy: Rationale and implementation. J Orthop Sports Phys Ther 2015; 45: 876-886.
- Hägglund M, Waldén M, Ekstrand J. Lower reinjury rate with a coach-controlled rehabilitation program in amateur male soccer: a randomized controlled trial. Am J Sports Med 2007. 35: 1433-1442.
- Carcia CR, Martin RL, Houck J, Wukich DK. Orthopaedic Section of the American Physical Therapy Association. Achilles pain, stiffness, and muscle power deficits: achilles tendinitis. J Orthop Sports Phys Ther 2010; 40: A1-A26.
- Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: Some aspects of basic science and clinical management. Br J Sports Med 2002; 36: 239-249.
- Silbernagel KG, Crossley KM. A proposed return-to-sport program for patients with midportion achilles tendinopathy: Rationale and implementation. J Orthop Sports Phys Ther 2015; 45: 876-886.
- Habets B, van den Broek AG, Huisstede BMA, Backx FJG, van Cingel REH. Return to Sport in Athletes with Midportion Achilles Tendinopathy: A Qualitative Systematic Review Regarding Definitions and Criteria. Sports Med 2018; 48: 705.
- Peters JA, Zwerver J, Diercks RL, Elferink-Gemser MT, van den Akker-Scheek I. Preventive interventions for tendinopathy: A systematic review. J Sci Med Sport 2016; 19: 205-211.
To cite this article
Comprehensive management of lower limb tendinopathies in athletes: advances and challenges
JOINTS 2024;
2: e931
DOI: 10.26355/joints_20244_931
Publication History
Submission date: 24 Jan 2024
Revised on: 27 Feb 2024
Accepted on: 20 Mar 2024
Published online: 15 Apr 2024